AI + Deep Brain Stimulation Could Unlock Paths to Depression Recovery
23 September 2023
A new deep brain stimulation (DBS) device, coupled with artificial intelligence (AI), holds potential to improve therapy for treatment-resistant depression. Researchers, utilizing this novel device capable of recording brain signals, have identified a biomarker associated with clinical signs of recovery from such depression. The findings from this preliminary study mark a significant step towards leveraging brain data to understand a patient’s response to DBS treatment.
Published in Nature, the study involved ten adults, with 90% showing significant improvement post-treatment. The biomarker helps distinguish mood fluctuations from severe depressive states, enabling personalized therapy adjustments. The findings, part of the BRAIN Initiative, represent a significant advancement in depression treatment, offering an objective measure akin to monitoring blood pressure or insulin levels for other diseases.
Depression, a pervasive mental health condition, has traditionally been challenging to monitor objectively, with clinicians relying on patients’ self-reported symptoms. However, this innovative research offers a potential game-changer in treating severe depression, providing a more objective measure akin to monitoring blood pressure for heart disease or insulin levels for diabetes.
“This study demonstrates how new technology and a data-driven approach can refine DBS therapy for severe depression, which can be debilitating. It’s this type of collaborative work made possible by the BRAIN Initiative that moves promising therapies closer to clinical use,” said John Ngai, Ph.D., director of the BRAIN Initiative.
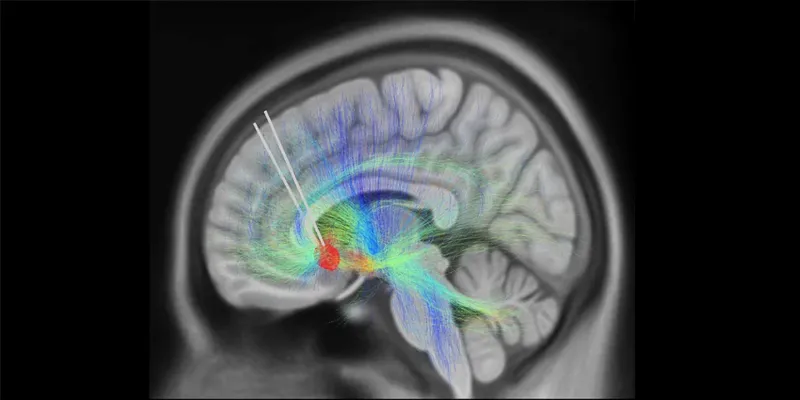
The study involved ten adults diagnosed with TRD, who underwent DBS therapy targeting the subcallosal cingulate cortex (SCC), a brain region integral to emotional behavior. DBS, an emerging therapy, involves implanting a thin metal electrode into specific brain areas to modulate neural activity through electrical impulses.

After 24 weeks of stimulation, a remarkable 90% of participants exhibited significant clinical improvement, with 70% achieving remission. The researchers, including Dr. Helen Mayberg from the Icahn School of Medicine and Dr. Christopher Rozell from Georgia Tech, utilized explainable AI to analyze the brain activity recorded by the DBS device. They successfully identified a distinct neural activity pattern or biomarker correlating with recovery from depression.
This identified biomarker is crucial in distinguishing transient mood fluctuations from more severe depressive states, thereby aiding clinicians in making informed adjustments to DBS therapy. The study also revealed that individual recovery trajectories were linked to the degree of preoperative damage within the targeted brain network, providing insights into the variability in depression treatment.
Furthermore, the research team employed AI tools to analyze changes in facial expressions, a potential behavioral marker, extracted from participant interviews. They discovered patterns that coincided with transitions from illness to stable recovery, offering an additional tool to track progress in DBS therapy.
According to Dr. Helen Mayberg, “Nine out of 10 patients in the study got better, providing a perfect opportunity to use a novel technology to track the trajectory of their recovery.”
The team is now validating their findings with a second cohort of patients and exploring the antidepressant effects of DBS using a next-generation device. This study represents a substantial advancement in early-stage DBS therapy for various mental disorders, including obsessive-compulsive disorder, post-traumatic stress disorder, and substance use disorder. The discovery of the biomarker and the insights gained into the multifaceted features of TRD pathology are motivating further research into the causes of variability in depression treatment.
Cingulate dynamics track depression recovery with deep brain stimulation
Abstract: Deep brain stimulation (DBS) of the subcallosal cingulate (SCC) can provide long-term symptom relief for treatment-resistant depression (TRD)1. However, achieving stable recovery is unpredictable2, typically requiring trial-and-error stimulation adjustments due to individual recovery trajectories and subjective symptom reporting3. We currently lack objective brain-based biomarkers to guide clinical decisions by distinguishing natural transient mood fluctuations from situations requiring intervention. To address this gap, we used a new device enabling electrophysiology recording to deliver SCC DBS to ten TRD participants (ClinicalTrials.gov identifier NCT01984710). At the study endpoint of 24 weeks, 90% of participants demonstrated robust clinical response, and 70% achieved remission. Using SCC local field potentials available from six participants, we deployed an explainable artificial intelligence approach to identify SCC local field potential changes indicating the patient’s current clinical state. This biomarker is distinct from transient stimulation effects, sensitive to therapeutic adjustments and accurate at capturing individual recovery states. Variable recovery trajectories are predicted by the degree of preoperative damage to the structural integrity and functional connectivity within the targeted white matter treatment network, and are matched by objective facial expression changes detected using data-driven video analysis. Our results demonstrate the utility of objective biomarkers in the management of personalized SCC DBS and provide new insight into the relationship between multifaceted (functional, anatomical and behavioural) features of TRD pathology, motivating further research into causes of variability in depression treatment.











Comments
No Comments Yet!