New Eye-Tracking Device, Promises Rapid and Accurate Autism Detection


Pediatrics |
7 September 2023
A groundbreaking eye-tracking device, approved by the FDA and named the EarliPoint Evaluation for Autism Spectrum Disorder, has shown potential in rapidly diagnosing autism in toddlers. The revolutionary device analyzes a child's gaze while watching videos, detecting signs of autism in 30 minutes. The newly published studies found the tool's accuracy to be on par with expert clinicians. This development offers hope for faster and more efficient autism diagnosis in the future.
Rapid and Accurate Autism Detection in 30 minutes
A new tablet-based device that tracks eye movements of children as they watch a video depicting a social interaction offers hope for faster autism spectrum disorder diagnoses. The tool, validated by two new studies published in JAMA and JAMA Network Open, demonstrates that it can detect autism in children aged 16 to 30 months as effectively as a specialist. By analyzing eye movements at 120 times per second, it identifies if a child's focus is on the interaction or elsewhere – a potential indicator of autism.
"Currently, autism is diagnosed by a highly trained expert over multiple hours of testing. Unfortunately there aren’t enough of these experts. The shortage of experts means that most specialty centers have long waiting lists. Identifying children with autism before age 3 is really important. The studies have shown that there are better long-term outcomes in children who are identified earlier," said the study’s lead author, Warren Jones, associate professor in the department of pediatrics at the Emory University.

Dr. Jones, emphasized the importance of early detection, given the plasticity of younger brains. The device, recently received FDA clearance and is undergoing further research for broader applications. With an average autism diagnosis age of 4.5 years, tools like EarliPoint could revolutionize early detection and treatment. In the larger study, which involved 1,089 children, the device displayed a sensitivity of 78% and specificity of 85.4%. Similarly, the secondary Phase III trial showcased a sensitivity and specificity of 81.9% and 89.9%, respectively, during the discovery stage, and 80.6% and 82.3% in the replication stage.
How does Eye-Tracking Device, EarliPoint work?
Autism Spectrum Disorder (ASD) diagnosis can be a prolonged journey, often taking over two years from initial suspicion to a confirmed diagnosis. However, this new device, approved by the FDA, is setting out to expedite this process. It operates by tracking a child's looking behaviour, determining whether they focus on faces or objects, and gauging the frequency of gaze shifts between focal points. Such behaviours are considered significant indicators of neurodevelopmental conditions like autism.
Impressively, findings from new investigations suggest that the device can detect autism with a level of sensitivity and specificity that matches the evaluations of experienced clinicians. These studies involved the examination of over 1,500 children across multiple specialty autism clinics.
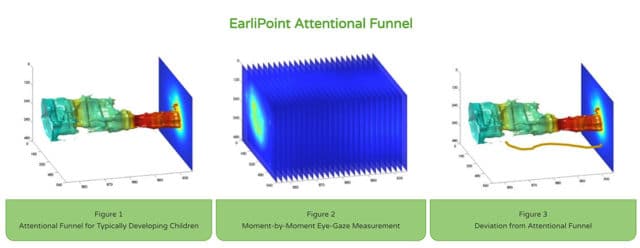
Why is this significant?
An early diagnosis of autism can pave the way for earlier interventions, thereby aiding in better language and social skill development. Moreover, the World Health Organisation (WHO) notes that 1 in 100 children are on the autism spectrum, underscoring the urgent need for faster diagnostic tools. The EarliPoint device uses Dynamic Quantification of Social-Visual Engagement (DQSVE) to measure the child’s moment-by-moment looking behaviour. This data is then compared to standard reference indices to determine levels of social disability, as well as verbal and non-verbal ability.
Looking to the Future
While the EarliPoint Evaluation shows considerable promise, researchers stress that it isn’t meant to replace expert assessments. Rather, it can provide supplementary data to assist clinicians in their diagnostic processes. Upon diagnosing autism, the data can also aid in crafting tailored support and intervention plans for each child. This development marks another leap in the ongoing advances in autism research and diagnostics, reinforcing the commitment to understanding and supporting those on the autism.
Abstract of the research
Eye-Tracking–Based Measurement of Social Visual Engagement Compared With Expert Clinical Diagnosis of Autism
Abstract: In the US, children with signs of autism often experience more than 1 year of delay before diagnosis and often experience longer delays if they are from racially, ethnically, or economically disadvantaged backgrounds. Most diagnoses are also received without use of standardized diagnostic instruments. To aid in early autism diagnosis, eye-tracking measurement of social visual engagement has shown potential as a performance-based biomarker. Results: Eye-tracking measurement of social visual engagement was successful in 475 (95.2%) of the 499 enrolled children (mean [SD] age, 24.1 [4.4] months; 38 [8.0%] were Asian; 37 [7.8%], Black; 352 [74.1%], White; 44 [9.3%], other; and 68 [14.3%], Hispanic). By expert clinical diagnosis, 221 children (46.5%) had autism and 254 (53.5%) did not. In all children, measurement of social visual engagement had sensitivity of 71.0% (95% CI, 64.7% to 76.6%) and specificity of 80.7% (95% CI, 75.4% to 85.1%). In the subgroup of 335 children whose autism diagnosis was certain, sensitivity was 78.0% (95% CI, 70.7% to 83.9%) and specificity was 85.4% (95% CI, 79.5% to 89.8%). Eye-tracking test results correlated with expert clinical assessments of individual levels of social disability (r = −0.75 [95% CI, −0.79 to −0.71]), verbal ability (r = 0.65 [95% CI, 0.59 to 0.70]), and nonverbal cognitive ability (r = 0.65 [95% CI, 0.59 to 0.70]). Conclusions and Relevance: In 16- to 30-month-old children referred to specialty clinics, eye-tracking–based measurement of social visual engagement was predictive of autism diagnoses by clinical experts. Further evaluation of this test’s role in early diagnosis and assessment of autism in routine specialty clinic practice is warranted.





Comments
No Comments Yet!