New Microdevice Offers Innovative Personalized Treatment for Brain Cancer
4 September 2023
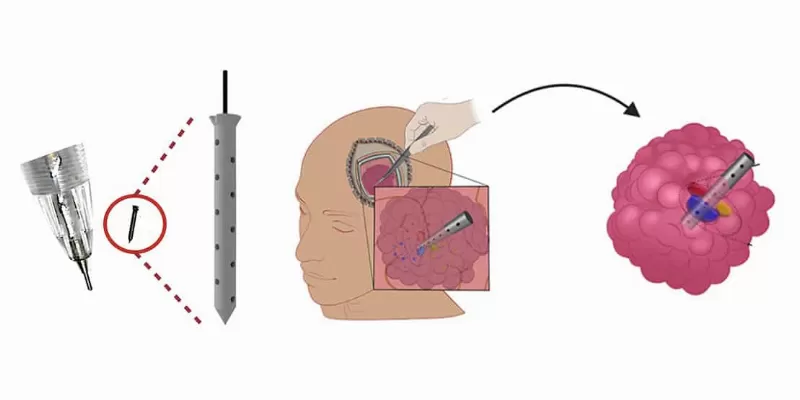
A cutting-edge microdevice, comparable in size to a grain of rice, promises a revolutionary approach to treating some of the most challenging forms of brain cancer. The pilot clinical trial demonstrated the device's ability to provide deep insights into drug effects on gliomas without any side effects. Implanted during standard surgery, the device releases doses of various drugs directly into the tumor. This method allows real-time drug testing within patients, marking a potential leap forward in personalized medicine.
In a pilot clinical trial published in Science Translational Medicine, the tool demonstrated its ability to provide unparalleled insight into the effects of drugs on gliomas without causing any side effects. Gliomas are among the deadliest brain cancers, afflicting roughly 20,000 individuals in the USA annually. Despite this high incidence, effective treatments have been elusive. A significant challenge in developing targeted therapies is the current limitations that prevent testing multiple drug combinations.
"In order to make the greatest impact on how we treat these tumors, we need to be able to understand, early on, which drug works best for any given patient. The problem is that the tools that are currently available to answer this question are just not good enough. So we came up with the idea of making each patient their own lab, by using a device which can directly interrogate the living tumor and give us the information that we need," said co-corresponding author Dr. Pierpaolo Peruzzi, MD, an assistant professor in the Department of Neurosurgery at Brigham and Women's Hospital.
The device functions by being temporarily implanted into a patient's tumour during standard surgery, where it releases small doses of up to 20 different drugs directly into the tumour. Post-implantation, the surrounding tissue is returned to the lab for in-depth analysis, enabling a precise assessment of drug impacts on both the tumour and its immediate environment.
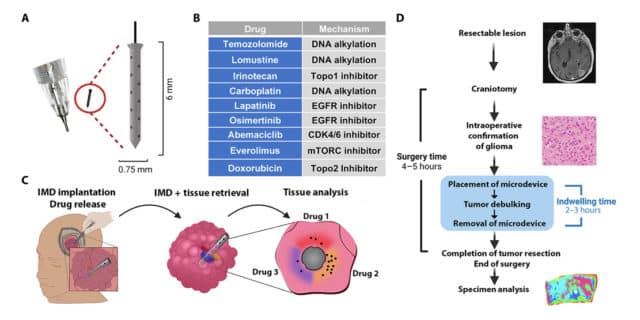
In the pilot study, six patients underwent this procedure with no adverse side effects reported. The data gathered provided a plethora of valuable insights, including molecular changes each drug induced in the cells. As researchers further refine the use of this device and interpret its data, they remain hopeful about its potential.
Dr. Peruzzi remarks, "This approach pushes the frontier of personalized medicine. Being able to conduct experiments right within the patient is groundbreaking, especially for a disease with currently limited treatment options."
The ongoing research reflects the next-stage version of the procedure, with patients receiving the device three days before their primary surgery. This advancement could mark a significant turning point in the treatment of gliomas, providing hope to thousands affected by this devastating condition.
Abstract of the research
Intratumoral drug-releasing microdevices allow in situ high-throughput pharmaco phenotyping in patients with gliomas
Abstract: The lack of reliable predictive biomarkers to guide effective therapy is a major obstacle to the advancement of therapy for high-grade gliomas, particularly glioblastoma (GBM), one of the few cancers whose prognosis has not improved over the past several decades. With this pilot clinical trial (number NCT04135807), we provide first-in-human evidence that drug-releasing intratumoral microdevices (IMDs) can be safely and effectively used to obtain patient-specific, high-throughput molecular and histopathological drug response profiling. These data can complement other strategies to inform the selection of drugs based on their observed antitumor effect in situ. IMDs are integrated into surgical practice during tumor resection and remain in situ only for the duration of the otherwise standard operation (2 to 3 hours). https://www.healthandmedicine.net/alzheimers-mysteries-3d-model-offers-new-treatment/ None of the six enrolled patients experienced adverse events related to the IMD, and the exposed tissue was usable for downstream analysis for 11 out of 12 retrieved specimens. Analysis of the specimens provided preliminary evidence of the robustness of the readout, compatibility with a wide array of techniques for molecular tissue interrogation, and promising similarities with the available observed clinical-radiological responses to temozolomide. From an investigational aspect, the amount of information obtained with IMDs allows characterization of tissue effects of any drugs of interest, within the physiological context of the intact tumor, and without affecting the standard surgical workflow.











Comments
No Comments Yet!