New Discovery: Are Cell Division Remnants the Hidden Facilitators of Cancer Spread?

Biotech |
19 October 2023
Researchers have identified a cellular fragment, previously considered cellular waste, that carries genetic information capable of transforming healthy cells into cancerous ones. This discovery suggests that these remnants might spread cancer throughout the body. Such insights might revolutionize cancer detection and treatment, opening new avenues for therapeutics.
Scientists from the Pasteur Institute in Paris and Harvard Medical School have made an unexpected discovery about a tiny cellular fragment, previously regarded as the "garbage bag" of cell division. This small structure, known as the midbody remnant, may play a crucial role in transforming healthy cells into cancerous ones.
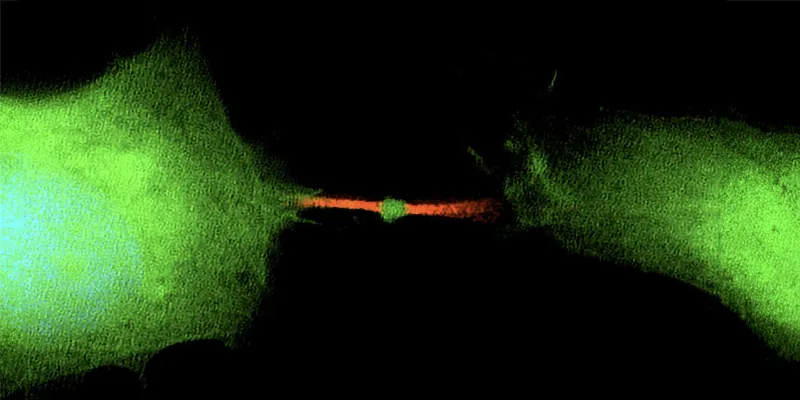
When a cell divides, a process known as mitosis, it results in two daughter cells. However, this division also produces a third, often overlooked, component: the midbody remnant. Contrary to previous assumptions that this was merely cellular waste, recent research has revealed that it carries vital genetic information.
“One cell divides into three things: two cells and one midbody remnant, a new signaling organelle. What surprised us is that the midbody is full of genetic information, RNA, that doesn’t have much to do with cell division at all, but likely functions in cell communication,” explains Ahna Skop, genetics professor from the University of Wisconsin–Madison.
A collaborative study, investigated the contents of these midbody remnants. Their findings, published in the journal Developmental Cell, indicate that these remnants could be vehicles for cancer to spread throughout the body. The RNA found within them is not just involved in cell division but also in activities that determine a cell's function, including the formation of cancerous tumors.
Interestingly, these remnants, which are a mere micron in size, can drift away from their origin, enter the bloodstream, and potentially influence other distant cells. If another cell absorbs a midbody remnant, it could start using the contained RNA as if it were its own blueprint. Past studies have shown that cancer cells are more likely than stem cells to incorporate a midbody and its RNA contents. The latter often ejects these remnants, possibly to retain their ability to develop into any cell type.
“People thought the midbody was a place where things died or were recycled after cell division. A midbody is a little packet of information cells use to communicate. The midbody remnant is very small. It’s a micron in size, a millionth of a meter,. But it’s like a little lunar lander. It’s got everything it needs to sustain that working information from the dividing cell. And it can drift away from the site of mitosis, get into your bloodstream and land on another cell far away,” Skop says.
Moreover, the team is working on advanced methods to isolate midbody structures from cell media or blood serum, which could enhance cancer diagnostics.
While these findings are groundbreaking, it's essential to remember that cancer's development involves multiple errors, and various mechanisms work to regulate unruly cells. Further research will explore the long-term fate of these RNA-loaded remnants and their exact role in cellular transformation.
This pioneering research received funding from organizations like the National Institutes of Health and the French Foundation ARC for cancer research.
Abstract of the research
The mammalian midbody and midbody remnant are assembly sites for RNA and localized translation
Summary: Long ignored as a vestigial remnant of cytokinesis, the mammalian midbody (MB) is released post-abscission inside large extracellular vesicles called MB remnants (MBRs). Recent evidence suggests that MBRs can modulate cell proliferation and cell fate decisions. Here, we demonstrate that the MB matrix is the site of ribonucleoprotein assembly and is enriched in mRNAs that encode proteins involved in cell fate, oncogenesis, and pluripotency, which we are calling the MB granule. Both MBs and post-abscission MBRs are sites of spatiotemporally regulated translation, which is initiated when nascent daughter cells re-enter G1 and continues after extracellular release. MKLP1 and ARC are necessary for the localization and translation of RNA in the MB dark zone, whereas ESCRT-III is necessary to maintain translation levels in the MB. Our work reveals a unique translation event that occurs during abscission and within a large extracellular vesicle.










Comments
No Comments Yet!