Modified mRNA Therapy Promises to Repair Heart Damage After Attack
14 September 2023
Researchers from the University of Alabama, have developed a modified messenger RNA (mRNA) that specifically targets heart muscle cells, or cardiomyocytes, to induce their regeneration after a heart attack. This treatment led to significant recovery in mouse and pig heart attack models by reducing dead heart tissue and improving the heart's pumping function without increasing arrhythmia risk.
The system, known as "cardiomyocyte SMRTs", enables the specific growth of new cardiomyocyte cells in the damaged area for less than four weeks. The findings are published in the journal Circulation Research. For the first time, researchers have showcased a modified mRNA therapy that uniquely targets cardiomyocytes, temporarily stimulating new cell growth for less than four weeks.
In animal tests, this method significantly enhanced recovery from acute heart attacks and reduced damaged heart tissue. While further research is needed before this treatment can be administered to patients, its potential significance is profound.
Following serious heart attacks, there's a significant risk of advancing to terminal heart failure, largely because of the reduced number of heart muscle cells which affects the efficiency of the heart's left chamber. This compromised heart condition, lacking self-repair capabilities, can further degenerate leading to congestive heart failure. For years, scientists have been exploring ways to encourage these cells to regrow post-heart attack.
The generation of new cardiomyocyte cells at the infarct scar, which is transient and specific to cardiomyocytes, is driven by a cardiomyocyte-specific modified mRNA translation system. This system, named "cardiomyocyte SMRTs" by the UAB researchers and developed by Dr. Zhang and his team, exclusively upregulates the expression of a targeted gene in cardiomyocytes. Modifying the mRNA allows a cell to interpret and convert the mRNA into protein. This breakthrough in modification made the COVID-19 RNA vaccines a reality.
The study's results represent a promising advancement in the potential treatment of heart attacks, particularly in the experiments conducted using the more clinically relevant large-animal models. The introduction of the SMRTs reduced the wall stress typically experienced after a heart attack by both promoting the generation of new cardiomyocytes and enhancing the survival of pre-existing ones.
Consequently, this led to a reduction in the size of the left ventricle infarct, improvement in left ventricle dilation and wall stresses, and a decrease in left ventricle hypertrophy.
Implications and Challenges Ahead
This significant stride could revolutionize the treatment of heart attacks. However, the treatment is not without its hurdles. While the study showed positive results when cardiomyocyte SMRTs were injected directly into the heart immediately following an induced heart attack, such an approach isn't feasible in typical clinical settings.
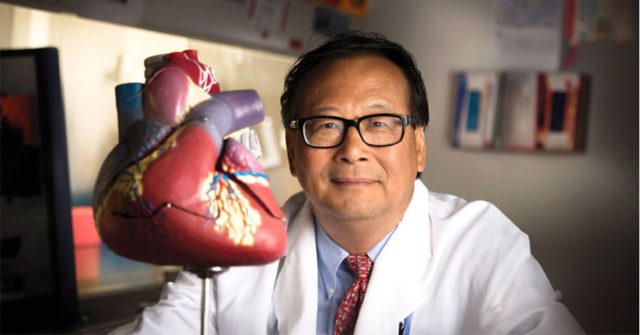
“Currently, direct intravascular or intracoronary injection of the cardiomyocyte SMRTs is unlikely to be maximally effective, because mRNA is rapidly degraded by RNase in the circulation. Although lipid nanoparticle-encapsulated modified RNA is FDA-approved and sufficiently stable to ensure that the modified RNA is taken up by cells, most systemically injected lipid nanoparticles home to the liver. To realize the full potential of modified RNA-based gene therapy for patients, different delivery methods such as cardiac-targeted lipid nanoparticles and different time points — weeks or months after heart infarct induction — will need to be developed and tested.” said Professor Jianyi “Jay” Zhang, chair of the UAB Department of Biomedical Engineering.
Abstract of the research
CCND2 Modified mRNA Activates Cell Cycle of Cardiomyocytes in Hearts With Myocardial Infarction in Mice and Pigs
Abstract: Experiments in mammalian models of cardiac injury suggest that the cardiomyocyte-specific overexpression of CCND2 (cyclin D2, in humans) improves recovery from myocardial infarction (MI). The primary objective of this investigation was to demonstrate that our specific modified mRNA translation system (SMRTs) can induce CCND2 expression in cardiomyocytes and replicate the benefits observed in other studies of cardiomyocyte-specific CCND2 overexpression for myocardial repair. Methods: The CCND2-cardiomyocyte-specific modified mRNA translation system (cardiomyocyte SMRTs) consists of 2 modRNA constructs: one codes for CCND2 and contains a binding site for L7Ae, and the other codes for L7Ae and contains recognition elements for the cardiomyocyte-specific microRNAs miR-1 and miR-208. Thus, L7Ae suppresses CCND2 translation in noncardiomyocytes but is itself suppressed by endogenous miR-1 and -208 in cardiomyocytes, thereby facilitating cardiomyocyte-specific CCND2 expression. Experiments were conducted in both mouse and pig models of MI, and control assessments were performed in animals treated with an SMRTs coding for the cardiomyocyte-specific expression of luciferase or green fluorescent protein (GFP), in animals treated with L7Ae modRNA alone or with the delivery vehicle, and in Sham-operated animals. Results: CCND2 was abundantly expressed in cultured, postmitotic cardiomyocytes 2 days after transfection with the CCND2-cardiomyocyte SMRTs, and the increase was accompanied by the upregulation of markers for cell-cycle activation and proliferation (eg, Ki67 and Aurora B kinase). When the GFP-cardiomyocyte SMRTs were intramyocardially injected into infarcted mouse hearts, the GFP signal was observed in cardiomyocytes but no other cell type. In both MI models, cardiomyocyte proliferation (on day 7 and day 3 after treatment administration in mice and pigs, respectively) was significantly greater, left-ventricular ejection fractions (days 7 and 28 in mice, days 10 and 28 in pigs) were significantly higher, and infarcts (day 28 in both species) were significantly smaller in animals treated with the CCND2-cardiomyocyte SMRTs than in any other group that underwent MI induction. Conclusions: Intramyocardial injections of the CCND2-cardiomyocyte SMRTs promoted cardiomyocyte proliferation, reduced infarct size, and improved cardiac performance in small and large mammalian hearts with MI. /










Comments
No Comments Yet!