First Patient Dosed in Phase 2 Trial of MB-105, CD5 CAR-T Therapy for T-Cell Lymphoma
March Biosciences announced the first patient has been dosed in its Phase 2 trial of MB-105, a first-in-class CD5-targeted CAR-T therapy for relapsed/refractory T-cell lymphoma. The trial aims to validate its safety and efficacy following promising Phase 1 results. MB-105 selectively targets malignant cells while preserving normal T-cells, addressing a major unmet need in T-cell cancer treatment.
The Phase 2 trial (NCT06534060) is a multi-center, single-arm, two-stage, open-label study designed to evaluate the safety and efficacy of MB-105 in patients with relapsed or refractory CD5-positive T-cell lymphoma. It will enroll approximately 46 patients across both stages. The trial will be conducted at leading cancer centers across the US, with the first patient dosed at The University of Texas MD Anderson Cancer Center.
“This represents a significant milestone in advancing MB-105 as a potential treatment option for patients with T-cell lymphoma who currently face extremely limited therapeutic choices,” said Sarah Hein, Co-Founder and CEO of March Biosciences. “CAR-T therapies have revolutionized the treatment of B-cell lymphomas and leukemias but have not successfully addressed the rarer T-cell lymphomas and leukemias. We are optimistic that this larger trial will further validate MB-105's potential to address the critical unmet needs of these patients.”
The Science Behind MB-105
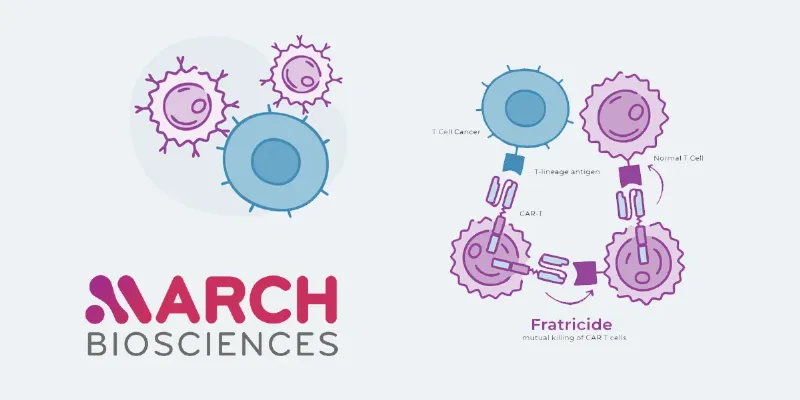
MB-105 is designed to target CD5, a surface antigen expressed in several T-cell malignancies, including T-cell lymphoma (TCL), T-cell acute lymphoblastic leukemia (T-ALL), chronic lymphocytic leukemia (CLL), and mantle cell lymphoma (MCL). The proprietary CAR construct allows for selective targeting of malignant T-cells, while sparing normal T-cell populations, a key innovation given the immunosuppressive risks inherent to most T-cell targeting strategies.
In a prior Phase 1 trial, MB-105 demonstrated durable remissions and a favorable safety profile in heavily pretreated patients. Notably, the therapy was successfully manufactured in 93% of attempts, and no grade ≥3 cytokine release syndrome or neurotoxicity events were observed—safety challenges that have hampered other CAR-T approaches.
Backed by Strong Financial and Regulatory Support
March Biosciences, a Houston-based biotech spun out of the Center for Cell and Gene Therapy at Baylor College of Medicine, is positioning MB-105 as a leading candidate in a therapeutic space long underserved. In 2024, the company secured a Series A financing round led by Mission BioCapital and 4BIO Capital, joined by notable investors such as Alexandria Venture Investments and Cancer Focus Fund.
The company has raised over $52 million to date, inclusive of venture financing, support from the Cancer Prevention & Research Institute of Texas (CPRIT), and the NIH SBIR program.
Further momentum came in January 2025, when the FDA granted orphan drug designation to MB-105, affording it potential incentives including tax credits, regulatory support, and seven years of market exclusivity if approved.











Comments
No Comments Yet!