IL-15 Agonist NKTR-255 Enhances CAR-T Therapy Effectiveness in LB-Cell Lymphoma

Clinical Trials |
ONCOLife |
11 December 2024
Nektar Therapeutics announced promising results from a Phase 2 study of its experimental drug NKTR-255, which improved six-month complete response rates in relapsed large B-cell lymphoma from 50% to 73%. It enhanced CAR-T cell expansion and durability, prompting some stable or partial responses to become complete responses. These early findings suggest NKTR-255 could help achieve more sustained remission in hard-to-treat cancers.
The promising outcomes, announced at the 66th Annual Meeting of the American Society of Hematology (ASH), come from a Phase 2 proof-of-concept study evaluating an investigational IL-15 agonist, NKTR-255, used alongside standard CAR-T cell therapy for patients with relapsed or refractory large B-cell lymphoma.
Dr. Sairah Ahmed, Director of the CAR-T Program at the University of Texas MD Anderson Cancer Center, emphasized the importance of these findings, highlighting the improved durability of response. “Patients achieving complete response at six months are highly likely to sustain remission beyond two years, translating into significantly improved progression-free survival,” she said.
In this trial, patients received either NKTR-255 or a placebo, beginning 14 days after their CAR-T infusion. Results showed that 73% of those treated with NKTR-255 achieved a complete response at six months, compared to 50% in the placebo group. Notably, two patients in the NKTR-255 group converted from stable disease or partial response to a complete response by the six-month mark, whereas no such conversions occurred in the placebo arm.
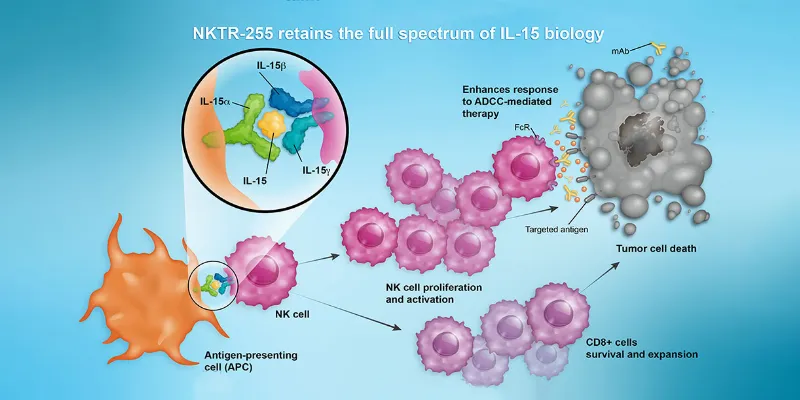
NKTR-255 works by activating and expanding natural killer (NK) cells and CD8+ T cells, thereby enhancing the effectiveness of CAR-T therapy. In this multicenter, double-blind study, 15 patients were randomized to receive either NKTR-255 or a placebo beginning 14 days after CAR-T infusion. The treatment boosted CD8+ CAR-T cell activity, demonstrating a 5.8-fold increase in area-under-the-curve kinetics during the critical 15-day post-administration window.
"This study is the first randomized trial demonstrating adjuvant treatment with NKTR-255 can enhance the durability of response to standard-of-care commercial CAR T-cell therapy by modifying CAR T-cell kinetics. These results contribute to the growing body of evidence demonstrating the broad applicability of NKTR-255 in combination with cellular therapies," said Dr. Mary Tagliaferri, Chief Medical Officer of Nektar.
Historical data from multiple trials and real-world analyses of commercial CD19-directed CAR-T products have shown six-month complete response rates between 41% and 44%. The improved rate seen with NKTR-255 could represent a step forward, potentially translating into longer progression-free survival for patients.
NKTR-255, a polymer-conjugated interleukin-15 (IL-15) agonist, targets a key immune system pathway. By stimulating and expanding the body’s natural killer (NK) and CD8+ T-cells, it aims to strengthen immunological memory and improve sustained anti-tumour responses.
Beyond the current CAR-T combination trial, NKTR-255 is also being investigated in other clinical settings, including a study evaluating its use alongside a tumour-infiltrating lymphocyte therapy for advanced lung cancer, and in combination with avelumab for bladder cancer.
Safety and Tolerability
Nektar reported that NKTR-255 was safe and well-tolerated in combination with FDA-approved CD19 CAR-T products, reinforcing its potential role in standard-of-care treatments.











Comments
No Comments Yet!