New Era in Biotech: Revolutionary Artificial Cells Could Mimic Life and Deliver Drugs

Biotech |
11 December 2023
Researchers from the University of Strathclyde have unveiled a groundbreaking method for creating synthetic artificial cells with properties akin to living cells. Published in Nature Chemistry, the study has the potential to transform the fields of synthetic biology and biotechnology. This innovation has the potential to be used in applications including drug delivery and tissue engineering.
The study marks a significant leap in synthetic biology, paving the way for advancements in medical science and blurring the boundaries between living and synthetic systems. By using synthetic materials, researchers have developed artificial cells that closely mimic the functions and structures of natural cells.
BioPISA: The Key to Artificial Life
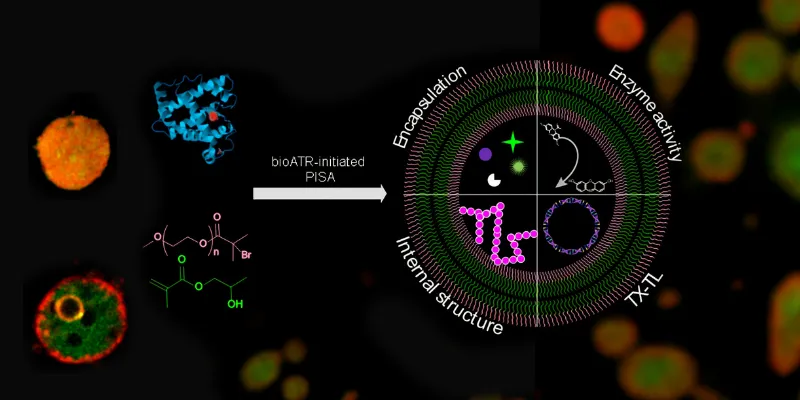
Led by Dr. Andrea Belluati, Prof. Nico Bruns, and Dr. Sètuhn Jimaja, the team pioneered a technique known as biocatalytic polymerization-induced self-assembly (bioPISA). This process involves the enzymatic synthesis of polymeric microcapsules, capable of encapsulating the contents of bacterial cells. What emerges are artificial cells that not only mimic the structural complexity of living cells but also demonstrate functional capabilities, such as protein production.
“This is a simple yet efficient way to prepare the artificial cells,” said Professor Nico Bruns of Strathclyde’s Department of Pure and Applied Chemistry and a co-leader of the study. “In future work, we aim to use proteins expressed in the artificial cells to catalyze further polymerizations, thereby mimicking the growth and replication of natural cells.”
Applications: From Drug Delivery to Tissue Engineering
These synthetic cells aren't just scientific curiosities; they hold immense potential for practical applications. Imagine microscopic structures, tailor-made to enhance chemical reactions, serve as hosts for synthetic biology pathways, and even assist in studying the origin of life. Their ability to produce a variety of proteins, including fluorescent markers and structural components like actin, opens up vast possibilities in drug delivery and tissue engineering.
A Step Closer to Mimicking Natural Cells
The creation of these artificial cells signifies more than just a technological triumph; it represents a conceptual shift in our understanding of life itself.
"Our study bridges a crucial gap in synthetic biology, merging the world of synthetic materials with enzymatic processes to create complex, artificial cells, just like real cells. This opens up new horizons in creating cell mimics that are not just structurally similar to biological cells but functionally competent as well," said Dr Andrea Belluati, Technical University of Darmstadt.
At the Edge of a New Revolution
Standing at the forefront of a new era in synthetic biology, this research marks the advent of a potential revolution. The ability to create cell-like structures that mimic growth, replication, and protein expression is not just an advancement in synthetic biology, but a transformative step towards blending biological and synthetic systems. This innovative international team's work heralds a future rich in biotechnological innovation and discovery.
Abstract of the research
Artificial cell synthesis using biocatalytic polymerization-induced self-assembly
Abstract: Artificial cells are biomimetic microstructures that mimic functions of natural cells, can be applied as building blocks for molecular systems engineering, and host synthetic biology pathways. Here we report enzymatically synthesized polymer-based artificial cells with the ability to express proteins. Artificial cells were synthesized using biocatalytic atom transfer radical polymerization-induced self-assembly, in which myoglobin synthesizes amphiphilic block co-polymers that self-assemble into structures such as micelles, worm-like micelles, polymersomes and giant unilamellar vesicles (GUVs). The GUVs encapsulate cargo during the polymerization, including enzymes, nanoparticles, microparticles, plasmids and cell lysate. The resulting artificial cells act as microreactors for enzymatic reactions and for osteoblast-inspired biomineralization. Moreover, they can express proteins such as a fluorescent protein and actin when fed with amino acids. Actin polymerizes in the vesicles and alters the artificial cells’ internal structure by creating internal compartments. Thus, biocatalytic atom transfer radical polymerization-induced self-assembly-derived GUVs can mimic bacteria as they are composed of a microscopic reaction compartment that contains genetic information for protein expression upon induction.










Comments
No Comments Yet!