Transforming Cancer Therapy by Taming the Chaotic MYC Protein Fueling 75% of Cases
6 January 2024
Researchers at UC Riverside have developed a groundbreaking peptide compound targeting MYC, a protein involved in 75% of human cancers. This novel approach, utilizing a stereodiversified bicyclic peptide library, overcomes the challenges posed by MYC's structureless form. This significant advancement in cancer research opens new avenues for therapy, promising a revolutionary impact on treating a wide range of cancers.
Understanding MYC: The Chaotic Protein
MYC, a protein integral to the transcription process in cells, usually functions by converting genetic information from DNA to RNA, and eventually into proteins. Min Xue, an associate professor of chemistry at UC Riverside, stated that MYC's activity is usually under strict control, but in cancer cells, it becomes excessively active and is not properly regulated.
“MYC is less like food for cancer cells and more like a steroid that promotes cancer’s rapid growth. That is why MYC is a culprit in 75% of all human cancer cases. It’s basically a glob of randomness. Conventional drug discovery pipelines rely on well-defined structures, and this does not exist for MYC. Peptides can assume a variety of forms, shapes, and positions. Once you bend and connect them to form rings, they cannot adopt other possible forms, so they then have a low level of randomness. This helps with the binding. We improved the binding performance of this peptide over previous versions by two orders of magnitude. This makes it closer to our drug development goals,” Xue said.
The Breakthrough: Bicyclic Peptide Library
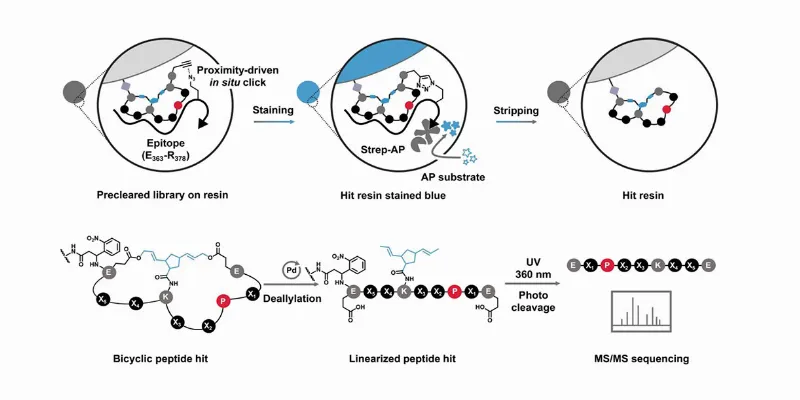
The UC Riverside team's remarkable breakthrough comes in the form of a peptide compound that binds to and suppresses MYC's hyperactivity. Their research, detailed in the Journal of the American Chemical Society and supported by the National Institutes of Health, describes a stereodiversified bicyclic peptide library, a novel and potent approach to tame this chaotic protein.
The innovation lies in the construction of the peptide. By altering the peptide’s rigidity and shape, the researchers have successfully improved its interaction with MYC. This new peptide demonstrates a sub-micro-molar affinity – an interaction strength nearing that of antibodies, which marks a significant improvement over previous versions.
Implications and Future Directions
The implications of this research are profound. By directly targeting MYC, new therapeutic avenues open up for a wide range of cancers. This novel approach to peptide library construction and the use of bicyclic peptides represent a significant leap in drug discovery techniques, with the potential to be applied to other challenging drug targets.
Furthermore, the creation of a stereodiversified library suggests the possibility of exploring new chemical spaces, crucial for finding effective binders for complex targets like MYC.
Currently, the focus is on enhancing the peptide’s ability to penetrate cells effectively, using lipid nanoparticles for delivery. Though still in the development phase, the potential for this peptide to change MYC's physical properties and inhibit its role in transcription activities holds immense promise.
A New Era in Cancer Treatment
As Prof. Xue aptly puts it, “MYC represents chaos, basically, because it lacks structure. That, and its direct impact on so many types of cancer make it one of the holy grails of cancer drug development. We are very excited that it is now within our grasp.”
This research marks a significant step forward in the battle against cancer, offering a novel and promising approach to targeting the MYC protein, a critical factor in a wide range of cancers.



Comments
No Comments Yet!