Revolutionary Dynein Discovery Could Curb Metastasis by Paralyzing Cancer Cells
28 October 2023
Penn State-led research has discovered that the motor protein dynein drives the movement of breast cancer cells, a key factor in metastasis. By targeting dynein, scientists believe they can halt cancer spread, offering an alternative to traditional treatments like chemotherapy. The study suggests a paradigm shift and could open pathways for new cancer therapeutics.
Scientists have unlocked new insights into the spread of breast cancer, a leading cause of death among cancer patients. Groundbreaking research led by Penn State has revealed that the motor protein dynein plays a pivotal role in the movement of breast cancer cells. The study, published in the journal Advanced Science, sheds light on potential therapeutic strategies.
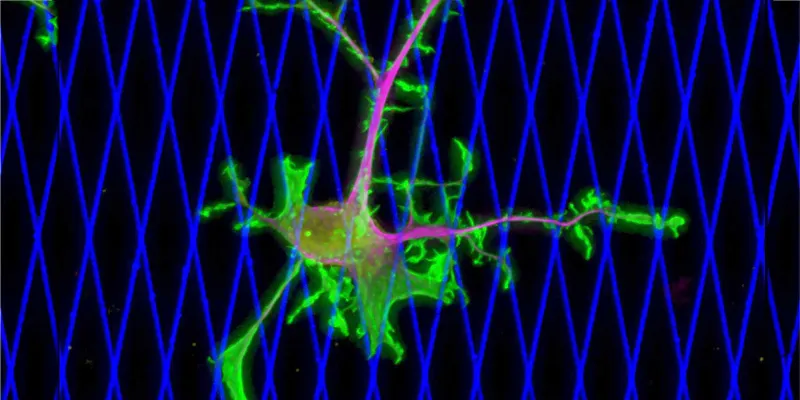
Metastasis, the spread of cancer cells to different parts of the body, is a particularly deadly aspect of cancer. The recent study delves into the mechanics underpinning how breast cancer cells move and invade healthy tissues. Remarkably, it was found that dynein is responsible for powering this movement.
Erdem Tabdanov, assistant professor of pharmacology at Penn State and a lead co-corresponding author of the study, remarked,
"This discovery marks a significant shift in our understanding. Previously, dynein's role in cancer cell movement was not recognized. Now, we understand that by targeting dynein, we could potentially halt the movement of these cells, thereby preventing metastasis."
Researchers used live microscopy and two innovative systems to study and observe the migration of live breast cancer cells. One system used a two-dimensional network of collagen fibers, which showed dynein's crucial role in cancer cell movement. The second, a three-dimensional model, mimicked soft tissue and further emphasized dynein's indispensable function in the metastatic spread of cancer cells.
Highlighting the potential benefits, Tabdanov added,
“The trick with chemotherapy is to kill the cancer cells slightly faster than the rest of the body — it’s a race against time. Chemotherapy causes a lot of damage to the body’s normal, healthy tissues while it is busy killing the cancer. If we instead contained the cancer, stopped it in its tracks, we could keep the healthy parts of the body healthy.”
Amir Sheikhi, who played a central role in the study, noted,
“Using these three-dimensional models that partially mimic a tumor, we discovered that if we block the dynein, the cancer cells cannot effectively move and infiltrate solid tissues. In both models, we found that dynein is extremely important for cell locomotion, which suggests a whole new method for cancer management. Instead of killing the cancer cells with radiation or chemotherapy, we are showing how to paralyze them. This is great news because you don't really have to kill the cells, which is a harsh approach that targets both cancerous and healthy cells. Instead, you just have to stop the cancer cells from moving.”
However, the road to clinical application is long. Human and animal trials have not yet been conducted, and further research is essential. Still, the team remains optimistic about the potential of their discovery. Sheikhi commented,
“I think these platforms could one day enable personalized medicine and personalized treatment for cancer and, hopefully, many other diseases.”
The research involved collaborations between Penn State’s Department of Chemical Engineering, Penn State’s College of Medicine, and other esteemed institutions and received support from various institutions, including the U.S. Food and Drug Administration and the National Institutes of Health.
Abstract of the research
Dynein-Powered Cell Locomotion Guides Metastasis of Breast Cancer
Abstract: The principal cause of death in cancer patients is metastasis, which remains an unresolved problem. Conventionally, metastatic dissemination is linked to actomyosin-driven cell locomotion. However, the locomotion of cancer cells often does not strictly line up with the measured actomyosin forces. Here, a complementary mechanism of metastatic locomotion powered by dynein-generated forces is identified. These forces arise within a non-stretchable microtubule network and drive persistent contact guidance of migrating cancer cells along the biomimetic collagen fibers. It is also shown that the dynein-powered locomotion becomes indispensable during invasive 3D migration within a tissue-like luminal network formed by spatially confining granular hydrogel scaffolds (GHS) made up of microscale hydrogel particles (microgels). These results indicate that the complementary motricity mediated by dynein is always necessary and, in certain instances, sufficient for disseminating metastatic breast cancer cells. These findings advance the fundamental understanding of cell locomotion mechanisms and expand the spectrum of clinical targets against metastasis.











Comments
No Comments Yet!