FDA Approves New Imaging System to Enhance Tumor Detection in Cancer Surgery
21 April 2024
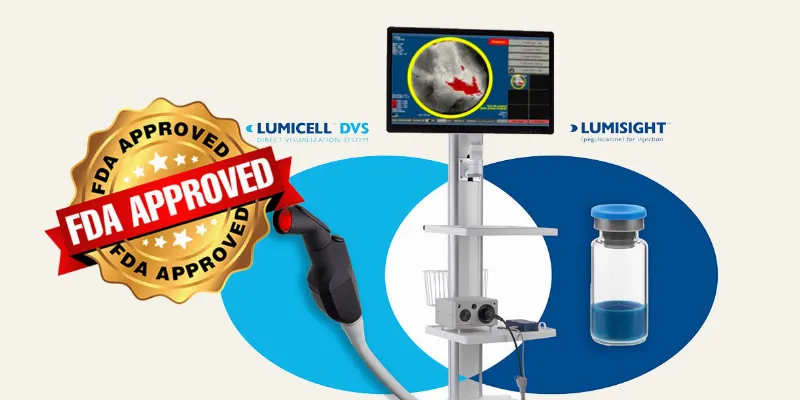
The U.S. Food and Drug Administration (FDA) has approved Lumicell's LumiSystem, a combination of a fluorescent imaging drug and a visualization device, to enhance the detection of residual cancer tissue during breast cancer surgeries. This innovative technology, proven in trials with over 700 patients, aims to improve surgical outcomes by reducing the need for second surgeries.
The LumiSystem enhances breast cancer treatment and surgical precision by allowing surgeons to scan the breast cavity with 84% diagnostic accuracy post-lumpectomy. This real-time detection and resection of residual cancerous tissue can significantly reduce the need for second surgeries, advancing both the effectiveness and safety of cancer surgeries.
Impact on Surgical Practices and Patient Care
Dr. Kelly Hunt, Chair of the Department of Breast Surgical Oncology at MD Anderson Cancer Center, highlighted the limitations of current intraoperative tools, which often fail to accurately identify the full extent of the tumor. This inadequacy can lead to as many as 36% of patients requiring a second surgery. Lumicell’s technology, aims to significantly lower this statistic by aiding in the complete removal of the tumor during the first surgery.
Innovative Technology for Cancer Detection
LumiSystem combines a fluorescent imaging drug, Lumisight (pegulicianine) administered intravenously, with a sophisticated imaging device, Lumicell DVS. This system is specifically approved for use in adults undergoing lumpectomy—a common breast-conserving surgery where the cancerous tumor and a small margin of surrounding tissue are removed. The technology aims to provide real-time feedback to surgeons, illuminating cancerous tissues that may otherwise be missed, potentially reducing the need for subsequent surgeries.,
“Up to 65% of the time, we do not find residual cancer in the second surgery and are left wondering if we performed an unnecessary surgery due to a false positive margin assessment or if the cancer was missed again in the second surgery,” said Dr Irene Wapnir, Breast Surgical Oncologist and Professor of Surgery, Stanford University School of Medicine.
Clinical Trials and Safety
The FDA’s approval was based on data from over 700 participants across five major studies. These studies demonstrated the system’s effectiveness in identifying residual cancer with high accuracy. However, the system is not without risks. The most common side effects observed were hypersensitivity reactions, including anaphylaxis, and chromaturia (abnormal urine color), occurring in a small percentage of patients.
With this approval, Lumicell is set to launch LumiSystem in the U.S. market soon. The company, known for its dedication to enhancing cancer surgery outcomes, also plans to explore further applications of its technology in other solid tumor surgeries.











Comments
No Comments Yet!