New Radioimmunotherapy Strategy Eradicates Cancer Stem Cells in Ovarian Cancer
1 August 2025
A novel radioimmunotherapy strategy has shown compelling efficacy in eliminating ovarian cancer stem cells in preclinical models, marking a major advance in efforts to address relapse and resistance in ovarian cancer. Published in the Journal of Nuclear Medicine, this study introduces a powerful new therapeutic avenue based on the radionuclide terbium-161 (¹⁶¹Tb), which demonstrated superior efficacy compared to the clinically established lutetium-177 (¹⁷⁷Lu).
Cancer stem cells (CSCs) represent a small but formidable subpopulation within tumors, characterized by self-renewal capacity, high tumorigenicity, and resistance to conventional therapies. Their persistence is linked to disease recurrence and metastatic spread. While efforts to develop CSC-targeted therapies have been ongoing, effective clinical strategies remain elusive.
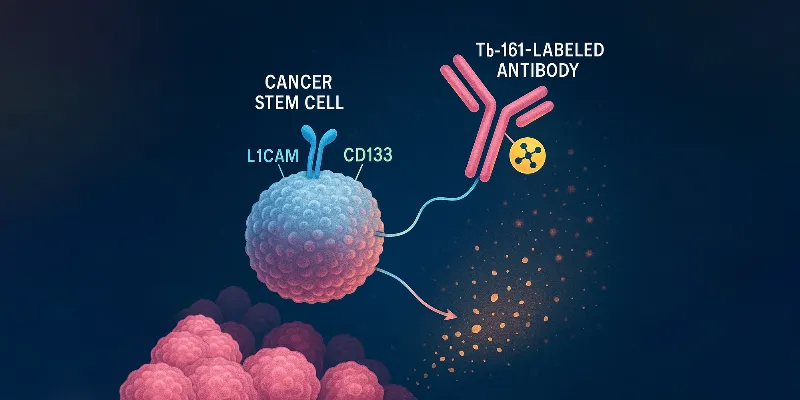
In this study, researchers from the Paul Scherrer Institute in Switzerland, led by Dr. Jürgen Grünberg and Dr. Tihomir Todorov, employed a radioimmunotherapeutic approach using radiolabeled antibodies directed against L1 cell adhesion molecule (L1CAM)—a validated biomarker for CSCs in ovarian cancer. The antibodies were conjugated with either ¹⁷⁷Lu or the emerging radionuclide ¹⁶¹Tb and tested in both in vitro assays and xenograft mouse models.
“Radioimmunotherapy enables precise, target-specific delivery of particulate radiation to cancer-associated antigens, while minimizing off-target accumulation and increasing tumor retention and irradiation, which makes it a promising choice for targeting CSCs,” said Dr. Jürgen Grünberg, “Our study sought to investigate the effectiveness of a new radionuclide Terbium-161 (161Tb) for eradicating CSCs due to emission of short-ranged conversion and Auger electrons—besides beta-minus particle—successfully eliminated ovarian CSCs in contrast to Lutetium-177 (177Lu).”
The findings were striking. While both radioconjugates showed comparable cellular uptake (50%–75% over 15 hours) and high immunoreactivity, ¹⁶¹Tb-labeled antibodies significantly outperformed their ¹⁷⁷Lu counterparts in eliminating CSCs and their progeny. Complete tumor eradication was achieved in all mice treated with ¹⁶¹Tb, whereas residual tumors were observed in the ¹⁷⁷Lu group.
“This signifies a pivotal step toward the translation of 161Tb-based therapies into clinical application,” noted Dr. Tihomir Todorov. “Targeted radionuclide therapies with 161Tb could support personalized medicine leading to advancements in cancer care including eradication of resistant CSCs and increased therapeutic efficacy alongside improved diagnosis, detection, and treatment monitoring.”
The study not only confirms the presence and tumorigenic capacity of L1CAM+/CD133+ CSCs in ovarian cancer but also underscores the therapeutic promise of ¹⁶¹Tb in precisely targeting these cells. Tumors derived from sorted CSCs reproduced the heterogeneity seen in patient samples, reinforcing the relevance of targeting this specific subpopulation.
From a broader clinical perspective, these findings may pave the way for more durable responses in patients with advanced or recurrent ovarian cancer. The authors suggest that ¹⁶¹Tb’s unique emission profile could also be exploited for imaging and treatment monitoring, supporting a more personalized and adaptive treatment approach.











Comments
No Comments Yet!