Vergent's VGT-309 Shows Promise in Real-Time Visualization of Tumors During Surgery

ONCOLife |
1 February 2024
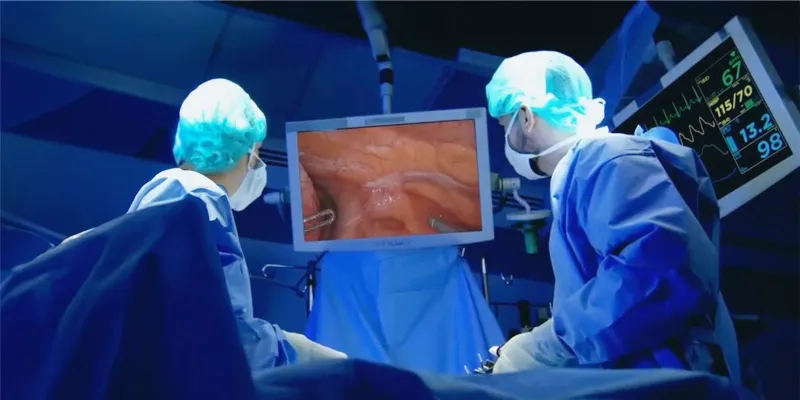
Vergent Bioscience's Phase 2 trial of VGT-309, a tumor-targeted fluorescent imaging agent, has demonstrated promising results in enhancing tumor visibility during lung cancer surgeries. Presented at the 60th Annual Meeting of The Society of Thoracic Surgeons, the trial underscores VGT-309's potential to increase the precision of minimally invasive and robotic-assisted surgeries.
The study findings indicate that VGT-309 could transform minimally invasive and robotic-assisted surgical procedures by enabling the real-time detection and removal of previously undetectable primary and metastatic tumors. This innovative advancement marks a pivotal moment in the fight against lung cancer, transforming surgical practices and patient outcomes.
John Santini, President and CEO of Vergent Bioscience, expressed optimism about the potential impact of VGT-309, stating, “The data presented today at STS 2024 suggest that VGT-309 could help support more confident and reliable removal of a wide breadth of cancers in the lung that would otherwise be missed during minimally invasive and robotic surgery, We look forward to the results from our ongoing, Phase 2 multi-center VISUALIZE study, which will provide further insight regarding the potential promising impact of VGT-309 for both cancer patients and surgeons.”
The Phase 2 efficacy study of VGT-309 assessed how frequently intraoperative molecular imaging (IMI) enhanced surgical outcomes in 40 individuals with suspected or proven lung cancer who were eligible for surgery. The primary efficacy endpoint was the percentage of patients experiencing at least one clinically significant event.
Clinically significant events were defined as the localization of lesions not identified by standard surgical techniques, the identification of synchronous and occult cancers, and inadequate surgical margins. Each patient in the study was administered VGT-309 preoperatively via intravenous infusion. Out of the 40 participants who received VGT-309 and underwent the standard-of-care surgical resection for suspected lung cancer, 17 (42.5%) experienced at least one clinically significant event.
VGT-309, in combination with NIR fluorescence imaging, effectively visualized a range of primary and metastatic tumor types, including adenocarcinoma in situ, invasive adenocarcinoma, lymphoma, colorectal cancer, neuroendocrine tumors, sarcomas, and squamous cell carcinoma. Notably, the study found VGT-309 to be safe and well-tolerated, with no reported infusion reactions or drug-related serious adverse events.
Commenting on the significance of the findings, Dr. Sunil Singhal, lead investigator and chief of the division of thoracic surgery in the Perelman School of Medicine, emphasized, “Minimally invasive surgery has become the standard of care for many cancer surgeries, but surgeons’ ability to see and remove all tumour tissue during these procedures is often hindered by poor visualization. The data from the VGT-309 Phase 2 efficacy study are encouraging, reinforcing the agent’s potential to help fill this critical gap.”
Vergent Bioscience is awaiting the results from its ongoing Phase 2 multi-center VISUALIZE study, looking forward to gaining further insights into VGT-309's potential impact on improving outcomes for both cancer patients and surgeons. Should it prove successful, VGT-309 could mark a significant advancement in surgical oncology, enhancing the precision and effectiveness of minimally invasive and robotic-assisted lung cancer surgeries. This advancement represents a collaborative success, supported by the National Cancer Institute of the National Institutes of Health.
About VGT-309
VGT-309, developed in Professor Matt Bogyo's Lab at Stanford University, is an innovative tumor-targeted imaging agent that enhances tumor visibility during various surgical procedures, including open, minimally invasive, and robotic-assisted surgeries. Administered through a pre-surgery infusion, it binds covalently to cathepsins, prevalent in many tumors, using indocyanine green (ICG), a dye compatible with existing NIR intraoperative imaging systems, to improve detection while minimizing background noise.










Comments
No Comments Yet!