Targeting eIF2A to Halt Melanoma: Study Reveals Key Driver of Cancer Cell Migration
2 August 2025
Researchers at the Centre for Genomic Regulation have identified eIF2A as a key protein that guides melanoma cells during metastasis. Known for its role in aiding protein synthesis under cellular stress, eIF2A also plays a critical part in helping cancer cells move through tissue. The study shows melanoma cells become dependent on eIF2A, suggesting a potential target to block metastasis selectively.
Published in Science Advances, the study uncovers a previously unknown function of eIF2A in directing the movement of melanoma cells. Rather than supporting protein synthesis, eIF2A stabilizes the centrosome—an essential structure for cellular orientation—allowing cancer cells to steer themselves through surrounding tissue.
“Malignant cells that metastasize need to make their way through tissues in order to invade proximal or distant organs. Targeting eIF2A could be a new strategy to impede melanoma breaking free and seeding tumours elsewhere,” says Dr. Fátima Gebauer, corresponding author of the study.
A New Role for a Familiar Protein
Melanoma is responsible for over 60,000 deaths globally each year. While it boasts a five-year survival rate of 99% in localized cases, that figure drops to around 35% once the cancer spreads to distant organs. Researchers have long sought to understand how melanoma cells gain the capacity to migrate and invade, in hopes of halting this deadly process.
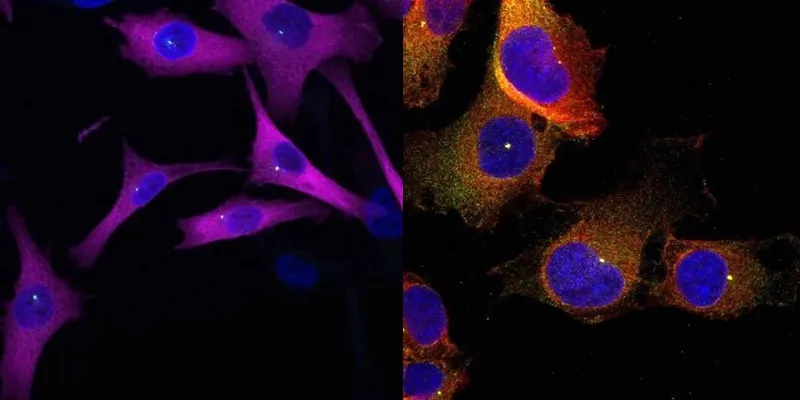
To investigate this, the team used two matched human cell lines—one non-tumoral and the other metastatic—to manipulate eIF2A expression. Surprisingly, silencing eIF2A had minimal impact on protein production. Yet the ability of cancer cells to migrate and form 3D tumor spheres was significantly impaired, suggesting that eIF2A’s role extended beyond translation.
The researchers then performed interactome profiling, isolating eIF2A and identifying its protein partners. Many were components of the centrosome—a cellular structure crucial for organizing microtubules and guiding directional movement. Further analysis revealed that eIF2A physically associates with the centrosome and is essential for its proper orientation during migration.
“The tail behaves like scaffolding cement, holding key parts of the melanoma’s cellular compass in place so that malignant cells can navigate their way out of the primary tumour,” said Dr. Jennifer Jungfleisch, the study’s first author.
A Translation-Independent Migration Driver
These findings mark a significant departure from the classical view of eIF2A. While it was previously linked to tumorigenesis through alternative translation initiation at non-standard start codons, this study shows that eIF2A can modulate cell behavior without altering protein synthesis.
Specifically, its disordered C-terminal tail—known for binding mRNA—proves essential for maintaining centrosome integrity and directionality during cell movement. Interestingly, the study also demonstrates that this function does not require active translation, suggesting a structural or scaffolding role for eIF2A at the centrosome. The protein’s involvement in centrosome composition and orientation appears to be a critical enabler of metastatic navigation, especially in transformed melanoma cells.
A Therapeutic Opportunity?
Crucially, eIF2A’s role in migration emerges only after malignant transformation, suggesting a therapeutic window where cancer cells—but not normal ones—are dependent on its activity. This specificity could reduce potential off-target effects in healthy tissues, a frequent obstacle in cancer drug development.
“In this field, many potential therapeutic targets prove either redundant or essential to normal cells, but the discovery of a protein that quietly makes itself indispensable only when cells become metastatic could be a rare catch. Any potential vulnerability counts,” concludes Dr. Gebauer.








Comments
No Comments Yet!