Milestone in Women's Health: Qvin's QPad Gains FDA Approval for Menstrual Blood Testing

FDA & EMA |
8 January 2024
In a groundbreaking advancement for women's health, the non-invasive menstrual blood test Q-Pad™ has received FDA clearance. Developed by the pioneering biotechnology company Qvin, this innovative test marks a pivotal moment for women. It enables millions of women to follow their health markers at home.
Qvin's visionary approach not only revolutionizes health monitoring but also addresses long-neglected issues in women's health, promising a paradigm shift towards more inclusive and accessible healthcare solutions. The clearance enables millions of women living with diabetes to monitor their A1c levels using laboratory tests performed on the Q-Pad.
Dr. Sara Naseri, Qvin Co-founder, highlighted the monumental shift in healthcare accessibility, stating, "With the first ever FDA-cleared menstrual blood health test, Qvin is paving the way to important new opportunities for women's health and this is just the beginning. We are simplifying routine testing, and freeing up resources that can be used on providing care and ultimately our goal is to make health care much more accessible."
Traditional blood testing methods typically involve invasive procedures conducted by medical professionals, and are often time-consuming and expensive. In contrast, Qvin's approach utilizes a common biological occurrence experienced by billions worldwide: menstruation.
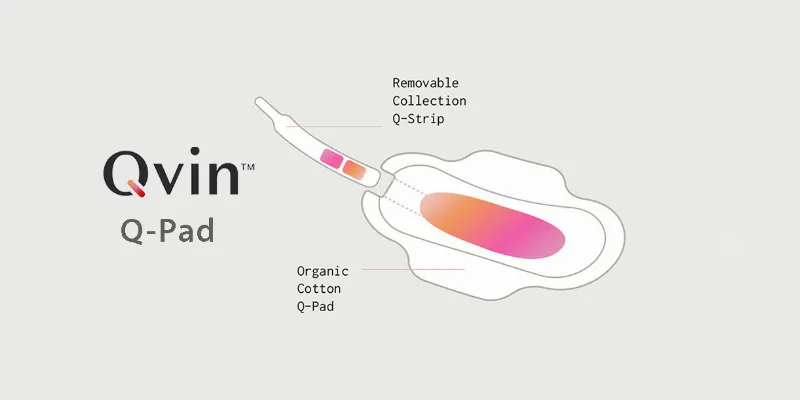
Q-Pad Operational Functionality
Each Q-Pad is equipped with a removable strip designed for collecting menstrual samples. These samples are then sent to a CLIA-certified (Clinical Laboratory Improvement Amendments) laboratory for clinical testing. Integration with the user-friendly Qvin app ensures swift and convenient delivery of test results.
The Qvin A1c Q-Pad Test Kit emerges as a crucial tool for monitoring blood sugar levels over a three-month period, significantly benefiting individuals with diabetes and even offering insights for non-diabetics regarding their blood sugar health impacts.
Søren Therkelsen, Qvin Co-founder, highlights the technology's potential to address neglected women's health issues stating: "Utilizing menstrual samples, the Q-Pad can address critical women's health issues that have historically been neglected. Because of our vertically integrated infrastructure, we will over time be able to deliver healthcare solutions at a significantly lower cost than traditional methods."
Furthermore, Qvin's collaborative research with institutions like Stanford University School of Medicine has validated the use of menstrual blood for monitoring a range of other biomarkers. These findings open the door to diagnosing and managing conditions often underdiagnosed or misdiagnosed, such as anemia, fertility issues, perimenopause, endometriosis, and thyroid health.
Dr. Paul Blumenthal of Stanford University, involved in multiple Qvin publications, emphasizes the novel and innovative nature of this research. He notes the potential for the Q-Pad in screening for reproductive hormones and even for Human Papilloma Virus (HPV), which is critical in global cervical cancer prevention efforts.
In conclusion, Qvin's FDA-cleared Q-Pad represents a significant leap in women's healthcare, offering a convenient, cost-effective, and less invasive option for health monitoring.
About Qvin
Qvin, named after the Danish word for women, aims to empower menstruating individuals by providing regular health insights for early detection and monitoring. Their product, the Q-Pad, is non-invasive and globally accessible, overcoming common obstacles like limited access, time, and financial constraints associated with traditional lab testing. Initially developed for HPV biomarker identification, Q-Pad now also tests for conditions like pre/diabetes, anemia, fertility, perimenopause, endometriosis, and thyroid health, enhancing health management and diagnosis accuracy.











Comments
No Comments Yet!