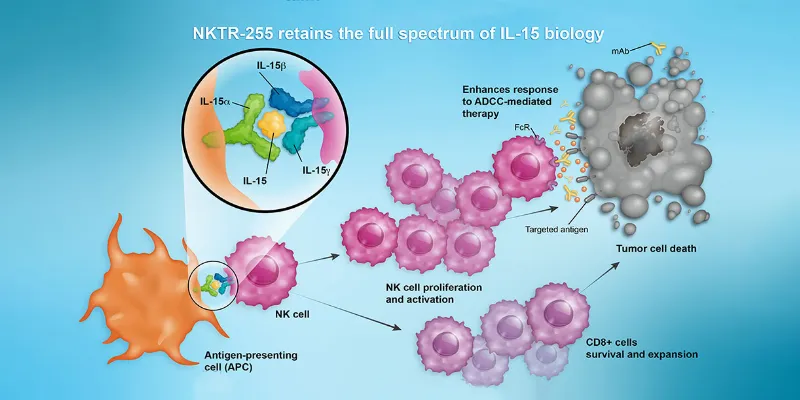
Tracy Mokwe
Health Tech Content Specialist
With over 3 years of experience as a medical laboratory scientist and writer, I am passionate about translating complex scientific information into clear and accessible content. My expertise lies in medical sciences, laboratory science and research, digital health, AI, and cybersecurity in healthcare.