Medtronic's PulseSelect PFA System Earns FDA Approval for Atrial Fibrillation Treatment

Medical Devices |
15 December 2023
Medtronic, has received FDA approval for its PulseSelect Pulsed Field Ablation System, designed to treat patients with paroxysmal or persistent atrial fibrillation. This innovative device is the first of its kind to combine pulsed field ablation, radiofrequency, and high-density mapping catheter technologies.
Medtronic plc marked an important success with the recent approval by the United States Food and Drug Administration (FDA) of its innovative PulseSelect Pulsed Field Ablation (PFA) System for treating both paroxysmal and persistent atrial fibrillation (AF). This development represents the first FDA approval for PFA technology and follows the system's earlier receipt of the European CE Mark in November. The approval follows successful results from two global studies.
Rebecca Seidel, SVP and President of the Cardiac Ablation Solutions business at Medtronic, articulated the significance of this achievement, stating:
"Launching the first FDA-approved PFA technology is not just a milestone; the PulseSelect PFA system is setting a new standard in safety for AF ablation with excellent efficacy and efficiency. It's a major step towards fulfilling our vision of providing disruptive electrophysiology solutions for patients."
Seidel emphasized that this milestone aligns with Medtronic's commitment to delivering disruptive electrophysiology solutions for patients, underscoring the system's advanced safety features and streamlined operational capabilities.
Atrial Fibrillation and Pulsed Field Ablation
Atrial fibrillation, affecting over 60 million people globally, is a prevalent yet undertreated heart rhythm disorder with potentially serious complications, including heart failure and stroke. Current ablation technologies carry risks of collateral damage due to thermal effects. PFA, as a breakthrough technology, utilizes pulsed electric fields to isolate pulmonary veins for AF treatment, potentially minimizing collateral structure damage due to its non-thermal cell death mechanism.
The PulseSelect PFA is a minimally invasive procedure that offers rapid, effective pulmonary vein isolation (PVI) while ensuring consistent and predictable energy delivery, coupled with enhanced catheter maneuverability. Its design allows for smooth integration into a clinician's preferred workflow. Supported by the PULSED AF study, the system demonstrated remarkable safety with a 0.7% safety event rate and achieved clinical success rates of 80% in both paroxysmal and persistent AF patients.
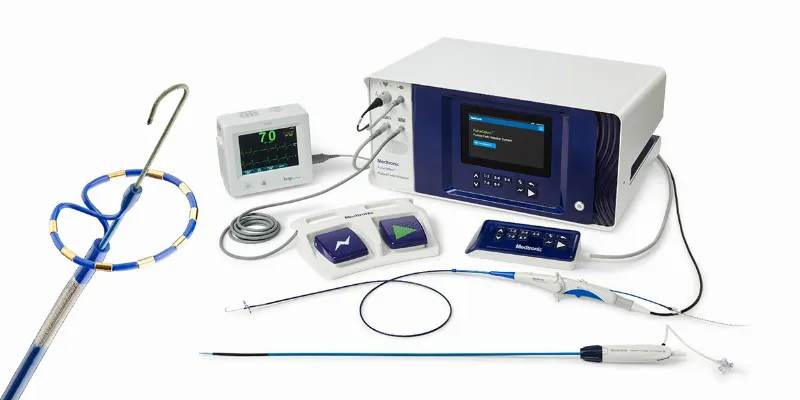
The PulseSelect™ PFA System consists of three components: the Sphere-9 PFA catheter, which delivers RF energy; the HD mapping catheter, which provides real-time feedback and navigation; and the PulseSelect™ software, which integrates all three components and enables intuitive mapping and ablation.
"The PulseSelect PFA system ushers the EP community to a new era of safe, effective, and efficient AF ablation that overcomes many challenges in our current practice," said Dr Amin Al-Ahmad, clinical cardiac electrophysiologist at St. David's Medical Center in Austin, TX and one of 67 global operators in the PULSED AF trial.
Key features of the PulseSelect PFA system
- Compatibility with any mapping system or use with just fluoroscopy, enhancing its versatility. - Built-in safety features like a phrenic nerve test pulse, providing a preliminary assessment of catheter proximity to the phrenic nerve before therapeutic application. - A nine-electrode catheter with fixed spacing, ensuring predictable, consistent electric field delivery for contiguous ablation. This catheter also facilitates pacing and sensing. - Enhanced maneuverability with a small, 9Fr bidirectional catheter, compatible with a 10Fr sheath, including the custom bidirectional FlexCath Contour™ sheath.








Comments
No Comments Yet!