Revolutionary Wireless Bioelectronic Implant Detects Early Signs of Organ Rejection

Digital Health |
9 November 2023
In a breakthrough that could redefine transplant medicine, researchers at Northwestern University have developed the world’s first internal bioelectronic implant. This wireless device provides continuous, real-time monitoring of transplanted organs and can detect early warning signs of organ rejection up to three weeks earlier than existing methods. It holds the potential to revolutionize post-operative care for millions of transplant patients worldwide and has a transformative effect on the monitoring of various organ health indicators.
Published in Science, the study marks a significant milestone in the convergence of materials science, bioengineering, and clinical medicine. In our exclusive interview, Professor Lorenzo Gallon, a transplant nephrologist at Northwestern Medicine who led the clinical portion of the research, described the innovation as a potential paradigm shift in patient care.
"The fear of rejection is a persistent shadow that looms over patients," he said. “Organ rejection can occur at any time; it may happen immediately after the transplant or years later, and often it occurs without warning, as patients may not experience any symptoms. Many of my patients live with constant anxiety, not knowing if their body is rejecting the transplanted organ.”
The World's First Internal Bioelectronic Device
The innovative wireless device was co-developed by John A. Rogers, Professor of Materials Science and Biomedical Engineering at Northwestern University, in collaboration with Professor Lorenzo Gallon.
This breakthrough could spell a new era in transplant medicine, offering patients a heightened chance of recovery and longevity with their new organs. The device, a forerunner in a potential series of internal monitoring tools, represents a critical milestone in the ongoing surveillance of organs.
Sharing his vision for the future impact of implantable devices, Professor Lorenzo Gallon noted that, with FDA approval, this innovation could pave the way for a new generation of devices capable of continuously monitoring vital signs. He added that the integration of continuous health data collection from within the body, combined with artificial intelligence for analysis, heralds a new era in the monitoring and treatment of diseases.
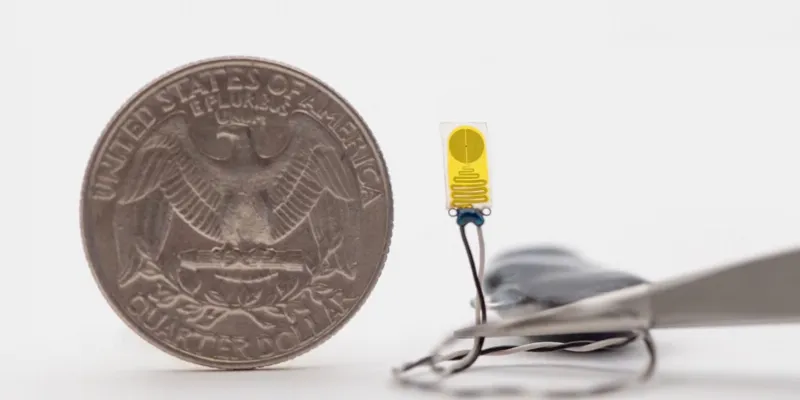
Tiny Soft Bioelectronic Implant
Describing the innovation as a 'tiny soft bioelectronic implant,' Professor Gallon elaborated on its functions.
"The device not only detects minute changes in temperature but also thermal irregularities indicative of inflammation, both key indicators of an organ's health. Remarkably compact, the sensor measures just 0.3 centimeters in width and 0.7 centimeters in length, with a thickness of only 220 microns. It's smaller than a fingernail. Yet, its ability to wirelessly transmit data to a smartphone or tablet provides patients and physicians with crucial information, potentially reducing the need for invasive biopsies or frequent hospital visits."
This device is set to address one of the most nerve-wracking aspects of organ transplants—the constant threat of rejection—by providing early warnings, which could lead to timely interventions.
Millions Worldwide Await Life-Saving Organ Transplants
"Currently, in the US alone, there are about 800,000 patients with end-stage renal disease, completely devoid of kidney function," he explained, highlighting the severity of the situation. "This dire scenario is further emphasized by the fact that out of the 110,000 people waiting for a kidney transplant, only approximately 25,000 receive one. The disparity in survival rates is stark and underscores the gravity of the situation."
Professor Gallon then delved into the statistics that reveal the harsh reality for those on dialysis. "For someone on dialysis, the five-year survival probability is about 48%, with a mortality rate nearing 60%," he said, comparing this to the significantly higher survival rate of 92% post kidney transplant. This contrast not only illustrates the urgent need for more transplants but also the life-changing impact they can have on patients.
The organ transplant process is a harrowing journey
Professor Lorenzo Gallon addressed the complexities and challenges of the organ transplant journey. He remarked,
"The organ transplant process is a harrowing journey, laden with uncertainty and risk, even after a patient has survived the long wait and received a transplant. While it can dramatically extend lives, the possibility of organ failure due to rejection is a constant risk—one that this device aims to mitigate."
Highlighting the delicate balance of the post-transplant period, Professor Gallon underscored that, while a transplant can significantly prolong life, the risk of organ failure due to rejection remains a persistent threat. "This is where our device comes into play, aiming to mitigate these risks," he added.
Lacking in Current Monitoring Methods
He emphasized the limitations of current monitoring methods.
“Most are blood-based, relying on markers like serum creatinine and blood urea nitrogen, which are neither specific nor sensitive enough. Normal creatinine levels can be misleading, often necessitating biopsies, which are invasive and not always feasible. On the other hand, new genomic markers are being introduced in clinical practice, but they suffer from poor predictability, high costs, and delayed results, making them impractical for quick clinical decisions.”
Addressing the numerous obstacles and challenges in monitoring organ health and rejection risk, Professor Gallon and his team have resolved to innovate a new approach to find solutions. His insights highlight the urgent need for breakthroughs in the field of organ transplant monitoring, aiming to improve the quality of life and increase the survival rates of thousands of patients.
This device, by addressing one of the most significant concerns in transplant medicine, represents a hopeful step towards improving patient outcomes and reducing the uncertainties that surround organ transplants.
He emphasized the device's capacity to detect temperature fluctuations throughout the day, signaling potential issues.
“Any rise in temperature is recorded and transmitted to an external receiver linked to the hospital's system. This can prompt immediate medical action, potentially leading to laboratory evaluations or targeted biopsies. Essentially, it reassures patients about their kidney's health and serves as an early warning system for doctors to conduct necessary tests.”
Professor Gallon anticipates that, upon applying for FDA approval, the device will be both affordable and transformative in measuring organ health.
''This could be the first among many devices that track physical indicators correlating with an organ's health or disease state,'' he added. He expressed excitement about the device entering clinical use. "It's a game-changer, offering physicians unprecedented levels of information and opening new research avenues. Once approved, it could become a treasure trove of transplant-related data. It's noninvasive, implanted during surgery, and could reveal unknown patterns or variations in diseases."
Concluding his interview, Professor Gallon emphasized the broader implications.
“We're on the cusp of a medical revolution with these types of internal devices. They could significantly impact how we manage and maintain transplanted organs, including kidneys, livers, and hearts. This device not only offers early detection and intervention possibilities but also prepares us for future advancements in transplantation. Early intervention is key in managing any disease process, and devices of this kind could be instrumental in transforming healthcare.”











Comments
No Comments Yet!