How SkinJect’s Microneedle-Patch Could Improve Skin Cancer Treatment Outcomes

ONCOLife |
2 July 2025
Basal cell carcinoma (BCC) accounts for nearly 80% of all skin cancer cases, yet treatment remains largely surgical, invasive, and aesthetically displeasig. In this exclusive interview, Dr. Raza Bokhari, CEO of Medicus Pharma, introduces SkinJect patch—a cellulose-based microneedle arrays delivering doxorubicin directly into lesions to trigger localized cell death and immune activation.
Dr. Bokhari discusses promising results from Phase 1, the ongoing 60-patient Phase 2 trial, regulatory milestones including a potential Fast Track designation, and how SkinJect may redefine the treatment landscape for BCC and other non-melanoma skin cancers worldwide.
Click the picture to view the PDF version: Pg 22-25.
Skin cancer is one of the most common cancer type. What challenges in current treatments inspired you to develop SkinJect?
Dr. Bokhari: Non-melanoma skin diseases are the most common cancers globally, and BCC is the most frequent, with nearly 5 million new cases annually in the U.S. alone. Fortunately, BCC is slow-growing and rarely causes serious illness or death. However, the standard treatment—Mohs surgery—is invasive, painful, costly, and limited to trained dermatologist in Mohs surgeons.
For decades, there has been a push to develop a non-invasive alternative, yet few have succeeded. That’s why we feel incredibly fortunate at Medicus Pharma to be not only first-in-class but also best-in-class with our non-invasive solution, SkinJect -offering a breakthrough treatment for non-melanoma skin cancers, and BCC in particular.
Mohs surgery is the standard treatment for BCC, but it can be invasive. How does SkinJect compare in terms of effectiveness, accessibility, and patient experience?
Dr. Bokhari: We’re developing a novel, patented treatment—an innovative combi-nation product originating from Carnegie Mellon and the University of Pittsburgh. Our technology uses dissolvable microneedle arrays loaded with doxorubicin, a well-known chemotherapy agent.
In our completed Phase 1 safety and tolerability study in 2021, we confirmed that doxorubicin was delivered safely with no serious adverse effects; we also saw comp-lete clinical responses on histological examination of resected specimens in 6 out of 13 patients -meaning cancer was cured in nearly half of them.
I’m a physician-turned-serial entrepreneur who has spent the past 30+ years in the U.S. building healthcare and life sciences companies. I began in diagnostics, then moved into therapeutics—launching startups and successfully exiting to strategic and financial buyers. For nearly the last decade, I’ve focused on clinical-stage therapeutic companies, using public markets to advance novel therapies. Medicus Pharma, our NASDAQ-listed company, is dedicated to progressing innovative therapeutic assets through clinical development and positioning them for exit near commercialization. Our lead asset, SkinJect, is designed to significantly disrupt the current treatment landscape for non-melanoma skin diseases.
We are now advancing a 60-patient Phase 2 trial across nine U.S. sites, which is doubleblinded, placebo-controlled, and includes three arms. In March 2025, we shared with the market findings of our positively trending interim data analysis, which showed complete clinical clearance of more than 60%
The interim analysis was conducted after more than 50% of the targeted 60 patients in the study were randomized. If SkinJect continues progressing as envisioned and gains approval, it could disrupt the current treatment paradigm by offering a relatively painless, cost-effective, and aesthetically favorable alternative to Mohs surgery. With millions affected by BCC, we believe this solution fills a major unmet need.
SkinJect’s technology delivers doxorubicin directly into the lesion while triggering an immune response. Can you explain how this dual mechanism contributes to both tumor eradication and long-term recurrence prevention?
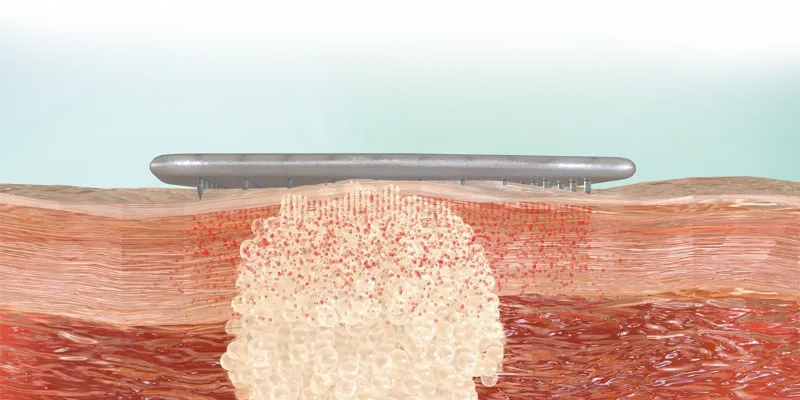
Dr. Bokhari: The SkinJect patch is a cellulose-based microneedle array designed with 400 microneedles in a 13mm x13mm active area. Each needle is 750 microns in length and tip-loaded with microdoses of doxorubicin, a well-known chemotherapeutic agent with over 30 years of established safety and efficacy.
This patch is applied directly over the lesion, where the microneedles penetrate the dermis and dissolve in the interstitial fluid, releasing the drug at the target site. This action triggers an immunogenic response through activation of the calreticulin pathway, ultimately causing cell death—both of malignant and healthy cells in the treated area.
In our Phase 1 study, which was primarily focused on safety, we observed complete clinical response in 6 out of 13 patients, with no serious adverse effects. Our ongoing Phase 2 trial is designed to validate these results with efficacy as the primary endpoint.
While recurrence data is still unknown, even Mohs surgery, considered the gold standard, has a 20% recurrence rate in some cases. The good news is that BCC is slow-growing, and whether treated surgically or with our patch, it can be managed effectively. In the US, morbidity and mortality from BCC are extremely rare when treated.
Your Phase 1 trial demonstrated a strong safety and efficacy profile. What key endpoints are you evaluating in the ongoing Phase 2 trial?
Dr. Bokhari: Our Phase 1 study primarily focused on safety and tolerability, the results showed that all 13 patients tolerated the treatment well, with no serious adverse reactions at the lesion site. As a secondary endpoint, we evaluated efficacy, and 6 of the 13 patients showed a complete clinical res-ponse, confirmed by excisional biopsy of the treated lesions.
Importantly, all six of these patients had the nodular subtype of BCC, which makes up about 70% of BCC cases and is typically treated with Mohs surgery. This encouraging result led us to focus our Phase 2 proof-of-concept trial solely on patients with nodular BCC. The ongoing 60-patient, double-blinded, placebo-controlled, 3-arm study includes a placebo group, a 100μg group, and a 200μg group.
Our goal is to replicate the efficacy seen in Phase 1, and we anticipate an interim data readout this quarter, which we plan to use to support a Type C meeting with the FDA by Q2 2025. We’re seeking Fast Track designation, with the intent to move into a pivotal trial of around 400 patients and eventually file a 505(b)(2) NDA for a new indication of doxorubicin. Our ambition is to become first-in-class—and ideally best-in-class—with a non-invasive treatment that could redefine the standard of care for BCC, aiming for commercial availability by the end of 2027.
How do you envision SkinJect fitting into mainstream dermatologic and oncologic practice?
Dr. Bokhari: We commissioned LEK to conduct a commercial analysis, including feedback from dermatologists in the U.S. and Europe, and response has been very positive regarding the adoption of our treatment—if it proves effective and becomes commercially available. We believe there will be minimal resistance to adoption from Mohs surgeons and dermatologists due to the strong value proposition and the way we have designed the treatment delivery.
In the U.S., when a patient is diagnosed with BCC, the average wait time for Mohs surgery is 6 to 8 weeks, sometimes even 4 to 6 months. Our SkinJect patch can be administered immediately in an office setting, with three 30-minute applications over two weeks. By week five, a visual inspection is done. If the lesion is cleared and the skin appears normal, surgery can be avoided. If not, the patient can still proceed with surgery, unharmed or delayed. We’re confident in positive adoption, especially as we aim to demonstrate strong clinical efficacy data this quarter and address a real unmet need.











Comments
No Comments Yet!