Beyond Visualization: The Promise of VGT-309 in Minimally Invasive Lung Cancer Surgery


ONCOLife |
22 May 2024
The investigational tumor-targeted fluorescent imaging agent VGT-309 may enable surgeons to see previously undetected or difficult-to-find tumors during surgery in real-time and help them ensure all tumor tissue is removed. In an exclusive interview with ONCOLife, Dr. John Santini, CEO and President at Vergent Bioscience, sheds light on VGT-309.
Dr. John Santini, discusses Phase 2 efficacy trial results revealed earlier this year at the 60th annual meeting of the Society of Thoracic Surgeons, and VGT-309’s potential impact on lung cancer surgery. Vergent Bioscience’s lead compound, VGT-309, is a tumor-targeted fluorescent imaging agent that enables surgeons to see previously undetected or difficult-to-find tumors in real-time, so they can ensure all tumor tissue is removed during surgery. Vergent Bioscience intentionally designed VGT-309 to fill existing deficits in tumor visualization.
Click the picture to view the PDF version: Pg 42-45.
Could you elaborate on VGT-309’s mechanism of action, particularly how its covalent binding to cathepsins enhances tumor visualization beyond traditional imaging agents during surgery?
Dr. John Santini: VGT-309 works by binding to cathepsins, a family of proteases that are highly overexpressed in a broad range of solid tumors. By binding tightly to these proteases, VGT-309 is retained at the tumor site during the surgical procedure, generating a sustained molecular signal that is visible when viewed with a near-infrared (NIR) camera. Because cathepsins are over-expressed in cancer as compared to normal cells, VGT-309 targets the tumor microenvironment while generating minimal signal in healthy tissue.
VGT-309 fluorescence is only visible with an NIR camera once the agent is bound and “activated” by the increased cathepsin protease activity in tumor tissue. This binding-dependent activity increases the signal produced by VGT-309 while minimizing the possibility of background fluorescence.
Considering the Phase 2 trial results, can you discuss VGT-309’s impact on lung cancer surgery and its comparison to current surgical imaging standards?
Dr. John Santini: Surgery is the first line of treatment for many solid tumors, but traditional open surgery can be traumatic and costly. Minimally invasive surgery (MIS), including robotic-assisted surgery, is increasingly utilized in lung cancer resection because it offers multiple benefits, including decreased tissue trauma, pain, blood loss, length of hospital stays, and postoperative complications.
However, a surgeon’s sight and ability to feel the tissue are often compromised during such procedures, making it more challenging to ensure all tumor tissue is removed. Additionally, surgeons are increasingly performing tissue-sparing (i.e., sub-lobar) lung cancer surgery, which can help maximize patients’ remaining lung function and reduce complications but increases potential for unseen tumor to be left behind. By illuminating tumor tissue during surgery, VGT-309 could provide missing piece for this emerging standard of care.
Why was lung cancer chosen as the initial focus for VGT-309’s evaluation, and are there plans to extend research to other solid tumor types?
Dr. John Santini: There is a tremendous need for improved visualization in lung cancer surgery. Lung cancer is the leading cause of cancer-related mortality in the United States, with more than 235,000 new cases anticipated for 2024. Approximately 25% of all U.S. lung cancer patients, equating to about 60,000 individuals, undergo lung cancer surgery every year. This number is expected to increase due to changes to USPSTF lung cancer screening criteria, which are likely to increase the number of lung cancers identified annually.
Also, the expanded use of MIS and robotic-assisted technologies for lung cancer surgeries has created a significant and growing need for better visualization during these surgeries, which can be curative when lung cancer is diagnosed early, and all tumor tissue is removed.
Vergent Bioscience is initially evaluating VGT-309 in this solid tumor given the breadth of patients who could benefit from improved, minimally invasive surgical approaches. Furthermore, as tissue-sparing techniques are becoming more prevalent in lung cancer surgery, the need for enhanced tumor visualization increases.
We’re pleased to share that in our clinical experience to-date, VGT-309 has visualized primary lung cancer and metastatic cancer in the lung including non-small cell lung cancer (NSCLC; adenocarcinoma and squamous cell carcinoma subtypes), large cell neuroendocrine carcinoma, colorectal, breast, prostate, carcinoid, sarcoma, and lymphoma.
While Vergent Bioscience is initially pursuing a clinical program in lung cancer, VGT-309 also has potential applications in surgery for patients diagnosed with other solid tumors, including colorectal, gastrointestinal, breast, ovarian, and prostate cancers. The estimated annual total number of surgeries worldwide for these additional indications is over 4 million, representing a significant opportunity for VGT-309.
How does VGT-309 integrate with current minimally invasive and robotic-assisted surgical technologies, and are there collaborations with equipment manufacturers plan-ned or underway?
Dr. John Santini: The imaging component of VGT-309 is the well-established NIR dye indocyanine green (ICG), which has been used by surgeons for decades. ICG is compatible with all commercially available NIR intraoperative imaging systems.
VGT-309 provides an added benefit to hospitals and health systems, many of which already have these NIR imaging systems in place. If VGT-309 is approved, hospitals will not need to purchase any specialized, and often expensive, hardware or software to use it.
Once the VISUALIZE study is complete, and assuming re-sults are positive, what are the forthcoming steps in VGT-309’s clinical development, including timelines for additional trials and potential regulatory approval?
Dr. John Santini: We expect to complete the multi-center Phase 2 VISUALIZE study later this year and follow it with a Phase 3 study. Assuming the results are positive, we would then plan to file an NDA for VGT-309 for cancer in the lung.
With advancements in tumor visualization, how do you foresee these innovations transforming surgical oncology and patient care?
Dr. John Santini: These advancements could potentially build surgeons’ confidence in MIS as well as help to achieve a complete oncologic resection by providing “molecular sight” to the surgeon.
These innovations create the potential that surgeons will not only be able to see the tissue, but also see the nature or biology of the tissue, to minimize normal tissue loss and ensure that all tumor tissue is removed. Looking ahead, the-se innovations have the potential to be used along with AI to provide surgeons with additional guidance to further optimize surgery.
How do VGT-309’s results to date compare to existing imaging technologies in lung cancer surgeries?
Dr. John Santini: Vergent Bioscience designed VGT-309 to fill the existing deficits in lung tumor and other solid tumor visualization. Currently available agents do not provide the same combination of features such as broad tumor target, covalent binding, molecular size, compatibility with commercially available NIR intraoperative imaging systems, and being activatable (i.e., fluorescence is ‘off’ until it hits its target and then turns ‘on’).
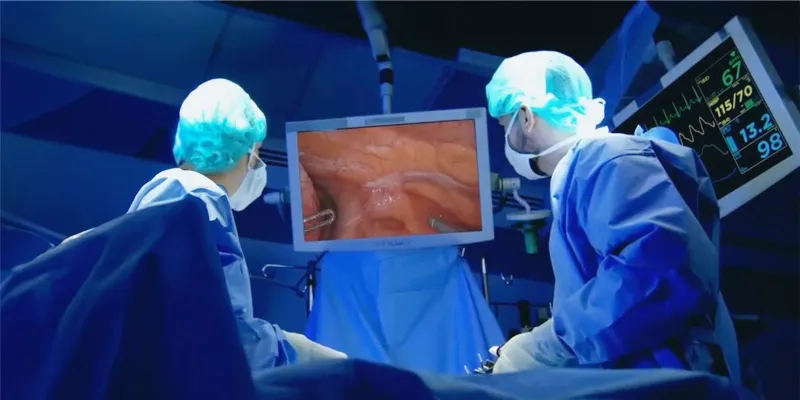
As noted previously, the imaging component of VGT-309 is the near infrared dye ICG, which has been used by surgeons for decades and is compatible with all commercially available NIR intraoperative imaging systems. A single-center Phase 2 efficacy study evaluated the frequency that intraoperative molecular imaging with VGT-309 improved surgical outcomes in 40 individuals with suspected or proven cancer in the lung who were eligible for surgery.
The primary efficacy endpoint was the proportion of patients with at least one clinically significant event (CSE), defined as localization of lesions not found by standard surgical techniques, identification of synchronous and occult cancers, and inadequate surgical margin. Of the 40 participants administered VGT-309 who underwent the standard-of-care surgical resection for suspected lung cancer, 17 (42.5%) had at least one clinically significant event.(1)
About John Santini
President and CEO of Vergent Bioscience, holds a Ph.D. in chemical engineering from MIT and a bachelor’s degree from the University of Michigan. A National Science Foundation Fellow under Professors Robert Langer and Michael Cima, Santini has 25 years of experience as a serial entrepreneur, inventor, and scientist in pharmaceuticals, drug delivery systems, biosensors, and surgical oncology products. Inspired by his early lupus diagnosis, he’s driven by a passion for science and a commitment to enhancing healthcare through technology innovation, aiming to improve health not as a physician but as a technology innovator.











Comments
No Comments Yet!