BioTrace: First FDA-Cleared Ultrasound Software for Liver Tumor Response Prediction

Medical Devices |
10 January 2024
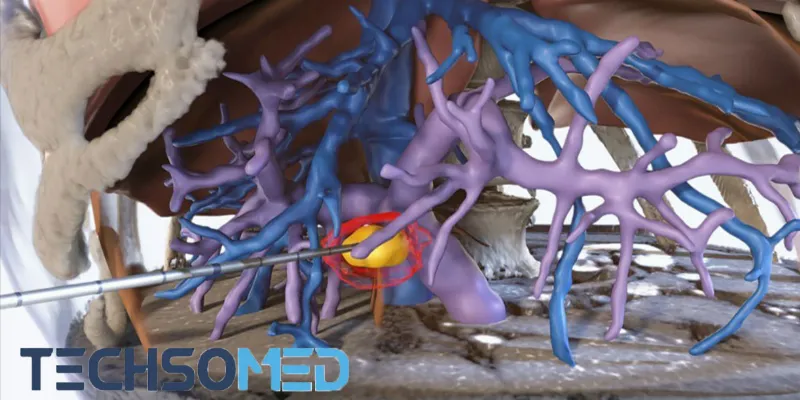
Techsomed Medical Technologies announces that the US Food and Drug Administration (FDA) has granted De Novo clearance for its BioTraceIO, an ultrasound-based liver ablation software. This innovative technology precisely predicts tissue response, significantly enhancing the accuracy of liver ablation procedures. As the first and only solution of its kind with FDA clearance, BioTraceIO aids physicians in liver tumor ablations.
Liver tumor ablation, a minimally invasive procedure using extreme temperatures to destroy cancer cells, has historically struggled with predicting outcomes based on ultrasound imaging. The introduction of BioTraceIO addresses this issue. This innovative software employs a unique computational algorithm to analyze ultrasound images during liver ablation treatment, providing real-time visualization and estimation of the ablated area.
Dr. Nami Azar, Professor of Radiology at UH Cleveland MC and principal investigator in BioTraceIO study, stated, “Imaging plays a crucial role in tumor ablation. While ultrasound serves as a cost-efficient and patient-friendly option for real-time visualization, there is a potential for enhanced visualization and continuous monitoring of ablation tissue response. BioTraceIO opens new possibilities in utilizing ultrasound for liver ablation procedures. I am excited to witness its impact in action and advancements it brings to our field.”
Rigorous Validation
BioTraceIO's performance was rigorously validated in a multi-center study in the USA involving 50 patients. The results demonstrated its superiority over immediate post-procedure CECT scans in estimating the ablation zone, closely aligning with observations from 24-hour CECT scans where maximum ablation zone expansion is observed. Notably, the software accurately predicts the ablation zone using only ultrasound imaging, providing essential information about size and shape that correlates with CT data.
The software, BioTrace.IO, complements Techsomed's VisAble.IO, an AI-powered product for ablation treatment planning and confirmation. This integrated solution aims to eliminate the guesswork typically associated with thermal ablation therapy, providing a comprehensive image-guided ablation visualization framework.
Yossi Abu, Techsomed's CEO and founder, emphasized the groundbreaking nature of the FDA's De Novo clearance stating: "Techsomed has taken a major step towards making our vision for image-guided ablation therapy a reality. The FDA's De Novo clearance is game-changing as we gear up to commercialize both BioTrace.IO and VisAble.IO across multiple market segments in the USA.”








Comments
No Comments Yet!