Molecular Trojan Horse: Peptide Nanotubes Offer a Path to Defeat Drug-Resistant Tumors

ONCOLife |
13 October 2025
Researchers at CiQUS have developed self-assembling peptide nanotubes that deliver doxorubicin directly into the nucleus of drug-resistant cancer cells, restoring the chemotherapy’s potency. The cyclic peptide–drug conjugates selectively target tumor membranes, bypass cellular efflux pumps, and sustain cytotoxic activity.
Drug resistance remains one of the most stubborn barriers in oncology. For patients whose cancers stop responding to chemotherapy, physicians often run out of options. Now, researchers at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS - USC), have found a molecular way to turn this problem on its head—literally—by reengineering how anticancer drugs enter and act within the cell.
Their strategy, published in ACS Applied Materials & Interfaces, relies on self-assembling peptide nanotubes—tiny hollow cylinders built from small cyclic peptides—to smuggle chemotherapy drugs directly into the nucleus of resistant cancer cells. The approach not only revives the potency of long-standing chemotherapies like doxorubicin but also circumvents the cellular mechanisms that normally deactivate or expel them.
A Molecular Trojan Horse
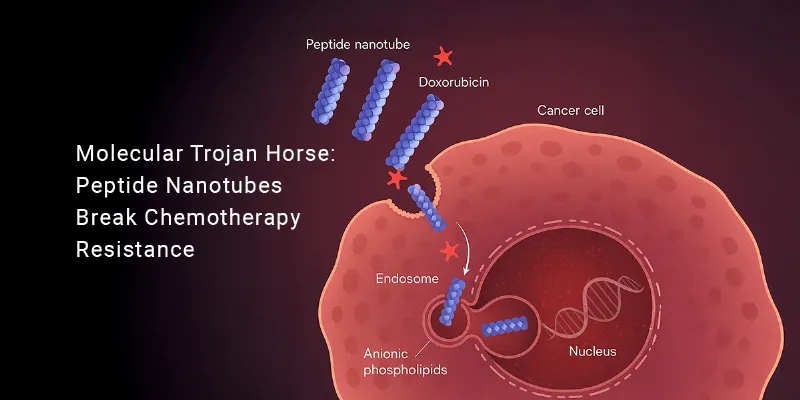
Doxorubicin, one of the most widely prescribed chemotherapeutic agents, works by intercalating with DNA and halting cell division. But many tumors learn to fight back by activating efflux pumps—molecular gatekeepers that eject the drug before it can reach the nucleus.
To outsmart this defense system, the CiQUS team, led by Dr. Juan R. Granja, designed cyclic peptides that naturally assemble into nanotubes capable of breaching resistant cells. These peptide rings alternate between right- and left-handed amino acids, giving them amphipathic and cationic properties—meaning they can interact powerfully with negatively charged, lipid-rich cancer cell membranes.
When these cyclic peptides are linked to doxorubicin, they form supramolecular nanotubes that act as molecular delivery vessels. Upon binding to tumor cell membranes, the conjugates are internalized through endocytosis. Once inside, their membrane-disrupting properties allow them to escape endosomes and release the drug—alongside the peptide—into the nucleus.
Targeting Cancer’s Weak Spot
Cancer cells are unusually rich in anionic phospholipids such as phosphatidylserine on their outer membranes—features rarely seen in healthy cells. The peptide nanotubes exploit this difference, displaying strong selectivity toward malignant cells while leaving normal tissue relatively unharmed.
Microscopy and biochemical assays revealed that these peptide–drug hybrids not only enter resistant cancer cells efficiently but also sustain the cytotoxic action of doxorubicin over longer periods. The peptides themselves reach the nucleus, where they may further enhance drug efficacy through synergistic interactions.
The self-assembling nature of these cyclic peptides gives them a unique advantage. They recognize and penetrate cancer cell membranes in ways conventional chemotherapies cannot, transforming the drug’s journey inside the cell.
Beyond Doxorubicin: A Platform for Next-Generation Therapies
The implications extend far beyond a single drug. By fine-tuning the peptide sequence, researchers believe they can design a library of nanotube systems adaptable to other chemotherapies—or even targeted biologics—that face resistance barriers.
The concept also opens the door to combination regimens, where peptide-based carriers could transport multiple agents through alternate routes, bypassing conventional resistance pathways. In future iterations, the nanotube system could be coupled with molecular sensors or imaging agents to track delivery and response in real time.
Toward Smarter Chemotherapy
While still at the preclinical stage, this peptide nanotube platform represents a conceptual leap in how clinicians might overcome drug resistance—by reimagining not the drug itself, but its mode of transport.
By merging supramolecular chemistry, peptide engineering, and cancer cell biology, the CiQUS team has demonstrated a new way to outmaneuver one of cancer’s oldest survival tricks. As the researchers note, optimizing the peptide sequence could yield even more potent derivatives—perhaps ushering in a new generation of smart chemotherapies that adapt to the molecular terrain of each tumor.











Comments
No Comments Yet!