Regulatory Approval Advances Pelareorep’s Virus Therapy for Pancreatic Cancer
15 January 2025
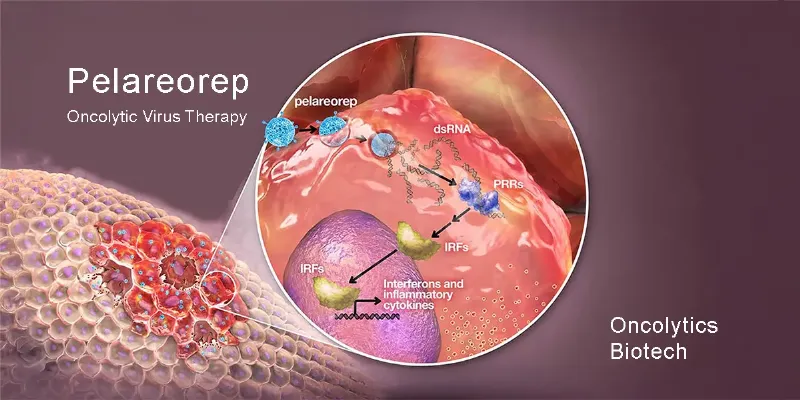
Oncolytics Biotech received approval from Germany\u2019s Paul-Ehrlich-Institute to advance Cohort 5 of the GOBLET study, evaluating pelareorep with mFOLFIRINOX, with or without atezolizumab, for metastatic pancreatic cancer. Promising early results highlight pelareorep\u2019s potential to improve outcomes for pancreatic cancer patients, with further data anticipated later this year.
The GOBLET study aims to explore the efficacy of pelareorep, an innovative oncolytic virus, in combination with modified FOLFIRINOX (mFOLFIRINOX) chemotherapy, with or without the immunotherapy drug atezolizumab (Tecentriq®), in newly diagnosed pancreatic ductal adenocarcinoma (PDAC) patients. With this green light, Cohort 5 can now enroll all 30 planned patients for its first stage.
Speaking on the development, Dr. Thomas Heineman, Chief Medical Officer at Oncolytics Biotech, expressed optimism: “Pelareorep has the potential to meaningfully improve outcomes for patients with metastatic pancreatic cancer. Encouraging tumor response rates observed in an earlier cohort of the GOBLET study underscore pelareorep's promise in this disease.”
Dr. Heineman also highlighted the study’s expanded focus, stating that GOBLET Cohort 5 explores pelareorep alongside mFOLFIRINOX, broadening the potential applicability of the therapy to a wider patient population.
Safety and Efficacy Insights
Early safety data will be presented at the 2025 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium later this month, with preliminary efficacy results anticipated later this year.
Design of GOBLET Cohort 5
Cohort 5 of the GOBLET study incorporates a Phase 1/2 design to evaluate two distinct regimens:
- Pelareorep + mFOLFIRINOX + atezolizumab
- Pelareorep + mFOLFIRINOX
The initial safety run-in included three patients to ensure tolerability, while the first stage involves enrolling 15 evaluable patients in each treatment arm. The co-primary endpoints are objective response rate (ORR) and safety. Should Stage 1 achieve its success criteria, Stage 2 will expand enrollment to an additional 17 evaluable patients per arm, with samples collected for advanced translational research.
About the GOBLET Study
The GOBLET study—a Phase 1/2 trial spanning 17 sites in Germany—is managed by AIO-Studien-gGmbH. It investigates pelareorep’s potential across multiple gastrointestinal cancer types. Cohorts include:
- PDAC patients treated with pelareorep, atezolizumab, gemcitabine, and nab-paclitaxel.
- Microsatellite instability-high metastatic colorectal cancer patients receiving pelareorep and atezolizumab.
- Third-line metastatic colorectal cancer patients, treated with pelareorep, atezolizumab, and TAS-102.
- Advanced anal cancer patients treated with pelareorep and atezolizumab.
- Newly diagnosed metastatic PDAC patients treated with pelareorep and mFOLFIRINOX, with or without atezolizumab.
Any cohort demonstrating efficacy in Stage 1 will proceed to Stage 2 for broader evaluation.
A Promising Future for Pancreatic Cancer Care
Pancreatic cancer remains one of the deadliest cancers worldwide, with limited treatment options and low survival rates. Pelareorep’s innovative approach—using an oncolytic virus to stimulate immune response—offers hope for transforming outcomes in this challenging field. Should the results of GOBLET Cohort 5 meet expectations, pelareorep could represent a breakthrough for a large population of metastatic pancreatic cancer patients in desperate need of new therapeutic options.











Comments
No Comments Yet!