Imlunestrant Shows Promising Results Alone and with Verzenio in ER+, HER2- Breast Cancer
The EMBER-3 trial showed that the new oral SERD, imlunestrant, significantly prolonged progression-free survival in patients with advanced ER+, HER2- breast cancer. In ESR1-mutated patients, it reduced the risk of progression or death by 38%. When combined with Verzenio, imlunestrant further lowered the risk of progression or death by 43% compared to monotherapy, indicating its potential as a more effective treatment option.
Eli Lilly and Company announced results from the Phase 3 EMBER-3 study, which evaluated the oral selective oestrogen receptor degrader (SERD) known as imlunestrant in patients with oestrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer. These results, shared at the San Antonio symposium (SABCS2024) and published in the New England Journal of Medicine, suggest a new, convenient oral treatment option for many patients.
"The median PFS observed in EMBER-3 is among the most compelling we've seen in CDK4/6 pre-treated ER+, HER2- advanced breast cancer patients and indicates a potential shift in the therapy options we provide for these patients, which are currently very limited. The benefit and safety profile of the imlunestrant and abemaciclib combination signal a potential new all-oral option for patients,” said Dr Komal Jhaveri, one of the study’s principal investigators and a researcher at Memorial Sloan Kettering Cancer Center.
Key Findings
- Imlunestrant as a single treatment: In patients whose cancers carried ESR1 mutations, imlunestrant as a standalone therapy significantly extended progression-free survival compared to standard endocrine therapy (such as fulvestrant or exemestane). Specifically, the median progression-free survival (PFS) was 5.5 months with imlunestrant versus 3.8 months with standard endocrine therapy. This equated to a 38% reduction in the risk of the disease worsening or leading to death.
- Imlunestrant plus Verzenio (abemaciclib): When combined with the CDK4/6 inhibitor abemaciclib (marketed as Verzenio), imlunestrant further improved outcomes. Across all patients—regardless of ESR1 mutation status—PFS reached 9.4 months on the combination therapy compared to 5.5 months for imlunestrant alone. This combination therapy reduced the risk of progression or death by 43%.
"EMBER-3 is the first Phase 3 trial to show benefit of combining an oral SERD with a CDK4/6 inhibitor for a patient population where an all-oral regimen would represent a meaningful advance. We're highly encouraged by these data for both imlunestrant as monotherapy and in combination with Verzenio, as well as the safety and tolerability profile, which demonstrate the potential for imlunestrant to be a meaningful new oral endocrine therapy option for patients," said Dr David Hyman, Chief Medical Officer at Lilly.
Potential Impact
The results are especially significant for individuals with ER+, HER2- advanced breast cancer who have already received standard hormonal treatments. Historically, these patients have had limited options, often requiring injections like fulvestrant. Imlunestrant is an oral drug, raising the prospect of a more convenient and potentially effective treatment option.
In the EMBER-3 trial, 874 patients were randomly assigned to receive either imlunestrant alone, a standard endocrine therapy, or the combination of imlunestrant and abemaciclib. The participants included those in their first line of advanced treatment—some having relapsed soon after completing adjuvant therapy—and those in the second line, following prior treatments for advanced disease.
While imlunestrant alone showed a significant benefit in patients with ESR1 mutations, the combination with abemaciclib was broadly effective, regardless of ESR1 or PI3K pathway mutation status, and maintained its advantage even in those previously treated with CDK4/6 inhibitors.
Side effects reported for the imlunestrant-abemaciclib combination were generally consistent with known profiles of drugs like fulvestrant when paired with abemaciclib. Common, mostly low-grade side effects included diarrhoea, nausea, neutropenia, and anaemia, with a low overall treatment discontinuation rate of about 6%.
What is the mechanism of action of imlunestrant?
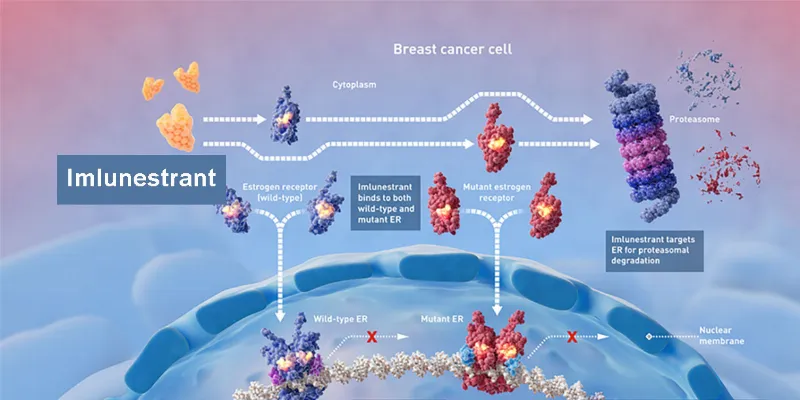
Imlunestrant is an orally bioavailable selective estrogen receptor degrader (SERD) that fully antagonizes both wild-type and mutant ERα, thereby preventing estrogen receptor-dependent gene transcription and cell proliferation. It consistently inhibits growth in ER+ breast cancer cells, regardless of ESR1 mutation status. In vivo, imlunestrant sustains inhibition of progesterone receptor expression, a marker of ER activity, resulting in robust single-agent anti-tumor effects. It induces tumor regressions in various xenograft and patient-derived xenograft models, both wild-type and ESR1-mutant. Additionally, combination studies demonstrate that imlunestrant is well-tolerated and its efficacy is enhanced when given alongside agents like abemaciclib, alpelisib, and everolimus.











Comments
No Comments Yet!