Palbociclib Combination Yields PFS Improvement in HR+, HER2+ Breast Cancer

Clinical Trials |
ONCOLife |
14 December 2024
The Phase 3 PATINA Trial shows that adding palbociclib (IBRANCE®) to standard maintenance therapy significantly extends progression-free survival (PFS) in HR+, HER2+ metastatic breast cancer. Presented at the 47th San Antonio Breast Cancer Symposium (SABCS), these findings demonstrate a more than 15-month gain in PFS. This key advance supports a new first-line strategy to improve patient outcomes and delay disease progression.
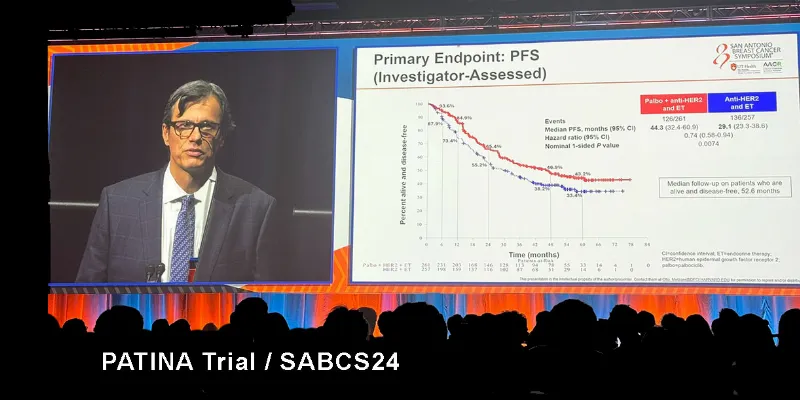
Key Results from the PATINA Trial
Sponsored by Alliance Foundation Trials (AFT) with support from Pfizer, the Phase 3 PATINA study involved patients receiving first-line maintenance therapy after initial chemotherapy. Participants were randomly assigned to receive either palbociclib combined with anti-HER2 and endocrine therapies or anti-HER2 and endocrine therapies alone.
“PATINA is the first large Phase 3 study to show the benefit of CDK4/6 inhibition in HR-positive, HER2-positive metastatic breast cancer,” said Dr. Otto Metzger, principal investigator of the trial for AFT and Medical Oncologist at the Dana-Farber Cancer Institute. “These results support the potential of this maintenance treatment to slow disease progression and improve clinical outcomes in this patient population.”
Striking Results
Median PFS improved from 29.1 months in patients receiving standard-of-care therapy alone to 44.3 months in those who also received palbociclib, representing a more than 15-month extension. This improvement was both statistically significant and clinically meaningful, suggesting a real potential benefit for patients.
Scientific Rationale and Background
About 10% of all breast cancers are HR+, HER2+, sometimes described as “double-positive” or “triple-positive” when also estrogen receptor-positive. Despite significant therapeutic advances, many patients eventually develop resistance to anti-HER2 and endocrine therapies, limiting long-term effectiveness.
“IBRANCE, the first CDK4/6 inhibitor, revolutionized the treatment of HR-positive, HER2-negative metastatic breast cancer, and has been prescribed to over 773,000 patients since its initial approval in 2015,” said Dr. Roger Dansey, Chief Development Officer, Oncology, Pfizer. “These results demonstrate that the addition of IBRANCE to standard of care shows promise as maintenance therapy in HR-positive, HER2-positive disease.”
Pre-clinical and earlier-phase research indicated that the cyclin D1-CDK4/6 pathway plays a critical role in driving this resistance, sparking interest in combining CDK4/6 inhibitors like palbociclib with standard anti-HER2 treatments. Earlier Phase 2 studies suggested that such combinations might enhance anti-tumor activity, and PATINA now provides strong Phase 3 evidence to support this approach.
Safety and Side Effects
The safety profile of palbociclib in PATINA was consistent with previous observations in HR+, HER2-negative disease, where palbociclib is already a standard first-line therapy. The most common side effects were blood-related, such as neutropenia, and non-blood-related effects like fatigue, mouth sores, and diarrhea. These were generally manageable and did not reveal any new safety concerns.
Future Implications
The extended PFS seen in PATINA is significant for patients and clinicians, potentially offering a new therapeutic approach for HR+, HER2+ metastatic breast cancer. Although palbociclib is currently approved in many countries for HR+, HER2-negative breast cancer, these new data may support broader use pending regulatory review.
The PATINA trial’s success also highlights the value of targeting multiple pathways simultaneously—combining CDK4/6 inhibition with anti-HER2 and endocrine therapies—to overcome resistance and prolong patient well-being. Pfizer plans to share the PATINA results with global regulatory agencies, aiming to expand options for patients facing HR+, HER2+ metastatic breast cancer.
About Palbociclib (IBRANCE)
IBRANCE is an oral inhibitor of CDKs 4 and 6,iii which are key regulators of the cell cycle that trigger cellular progression.iv,v In the U.S., IBRANCE is indicated for the treatment of adult patients with HR+, HER2- advanced or metastatic breast cancer in combination with an aromatase inhibitor as initial endocrine-based therapy in postmenopausal women or in men; or with fulvestrant in patients with disease progression following endocrine therapy.











Comments
No Comments Yet!