The VAPOR 2 Trial and the Potential of Water Vapor Ablation in Prostate Cancer Treatment

ONCOLife |
2 October 2025
Currently being evaluated in the pivotal VAPOR 2 clinical trial, the Vanquish system offers a minimally invasive approach that targets cancer with precision while preserving surrounding tissue. With FDA Breakthrough Device Designation status and promising early data, this novel technology could transform treatment standards for prostate, kidney, and bladder cancer in the years ahead. In this ONCOLife interview, Michael Kujak, CEO of Francis Medical, discusses a bold innovation in prostate cancer care: water vapor ablation.
Click the picture to view the PDF version: Pg 24-27.
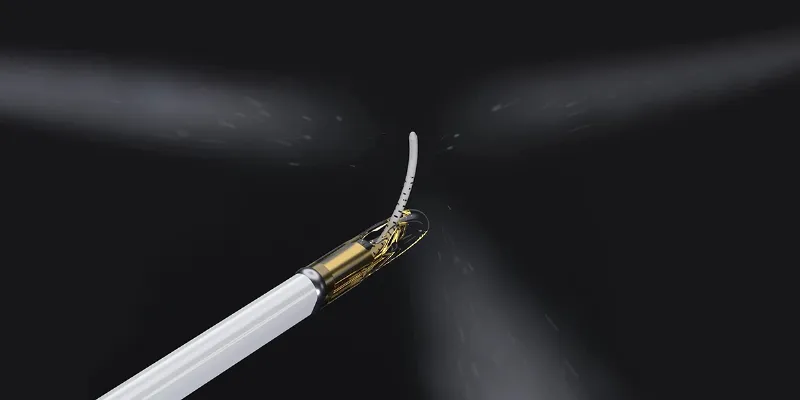
Water vapor may seem an unlikely ally in the fight against prostate cancer, but in the hands of medtech innovators, it’s becoming a precision tool with the potential to reshape treatment standards. The Vanquish system delivers thermal energy via convective vapor, ablating tumor tissue while potentially sparing critical structures.
In the evolving field of urologic oncology, innovation often means walking a tightrope—balancing efficacy with quality of life. For Michael Kujak, the future of prostate cancer care may lie not in removing or irradiating the entire gland, but in precisely ablating only what needs to be treated—using vapor.
“Our goal is to develop minimally invasive therapies that aggressively target cancerous tissue while preserving the patient’s quality of life,” Kujak explains. “Our guiding principle is simple: creating therapies that are tough on cancer, gentle on patients.”
At the heart of this mission is the Vanquish Water Vapor Ablation System—a novel approach currently being evaluated in the VAPOR 2 pivotal clinical trial. With promising early data, Francis Medical is now looking to position Vanquish as a first-line treatment for prostate cancer.
“The Vanquish system harnesses the energy released when sterile water is converted into vapor,” Kujak explains. “This convective therapy targets and destroys cancerous tissue while helping preserve surrounding healthy structures.”
Unlike traditional conductive ablation techniques—such as cryotherapy or focal laser ablation—Vanquish uses convective water vapor energy. This allows heat to move more selectively, traveling through the interstitial spaces between cells and releasing thermal energy upon condensation. This triggers apoptosis, the natural death of cancerous cells, within 24 hours.
“Importantly, the prostate’s natural boundaries, like the surgical capsule, contain the vapor within the targeted area,” Kujak notes. “This helps protect vital structures such as the urinary sphincter and the nerves responsible for erections.”
This mechanism stands in stark contrast to conventional therapies, where energy can extend beyond the prostate and affect nearby nerves and blood vessels, increasing the risk of urinary incontinence and erectile dysfunction. Launched in late 2023, the VAPOR 2 study builds directly upon the company’s earlier VAPOR 1 trial, which demonstrated no serious adverse events and an 87% success rate in eliminating clinically significant prostate cancer. VAPOR 2 is treating 235 men with intermediate risk prostate cancer.
“We’re targeting FDA 510(k) clearance in late 2025, based on six-month biopsy results and one-year safety data in over 100 patients,” Kujak says. “Then we plan to pursue Premarket Approval (PMA) in 2028, supported by three-year biopsy data and extended safety outcomes.”
Building on a Breakthrough
“Achieving Breakthrough Device Designation in July 2023 was a major milestone,” says Kujak. “At that time, there was no clear regulatory pathway for a technology like ours to seek a prostate cancer indication.”
The designation has had a tangible impact by streamlining communications with the FDA through expedited “Sprint” meeting discussions with FDA and elevating awareness of the technology within the agency. This early alignment is helping Francis Medical move swiftly toward market entry while maintaining scientific rigor.
“This designation has not only validated our approach but also provided a streamlined route to potentially bring a first-of-its-kind therapy to prostate cancer patients.”
So how does water vapor ablation differ at a fundamental level from heat- or cold-based treatments?
“When water converts to steam, this phase shift causes a dramatic increase in energy—to 540 Cal/gram,” Kujak explains. “When this vapor condenses inside the prostate, the energy is released onto the cell walls, disrupting the membrane and leading to cell death over approximately 24 hours.”
Crucially, convective energy, unlike conductive, doesn’t spread uncontrollably. “It’s similar to how wind hits a sail, it doesn’t pass through but bounces off. That’s how the fibrotic prostate capsule contains the vapor,” he says. “This mechanism helps minimize damage to surrounding structures.” This selectivity is what could make Vanquish a game-changer in minimizing side effects—a key unmet need in prostate cancer management.
Beyond Prostate Cancer
While prostate cancer is the company’s first major indication, the potential of water vapor therapy extends to other urologic malignancies.
“Adapting the technology for kidney and bladder cancer presents similar challenges to our transition from treating BPH to prostate cancer,” Kujak explains.
Bladder treatments will remain transurethral, while kidney applications will be performed percutaneously. The company has already begun preliminary bench and extirpated studies to refine these approaches.
First-Line and Future-Ready
Despite decades of progress in oncology, prostatectomy and radiation still dominate prostate cancer care—often at the expense of patient quality of life.
Kujak sees an opportunity to shift the standard. “Our strategy is to establish Vanquish as the first-line choice by demonstrating that we can achieve oncologic outcomes similar to radical prostatectomy and radiation, while reducing the unwanted side effects associated with these treatments.”
This positioning reflects a broader trend in oncology toward organ-preserving, image-guided therapies, especially as imaging, robotics, and intraprocedural guidance technologies continue to improve.
A New Standard of Care
“I believe that Vanquish will change the way prostate cancer is treated,” Kujak says. “By moving the standard of care away from removing or radiating the entire prostate to treating only the areas where imageable disease has been identified.”
This shift, however, won’t happen in isolation. “We cannot accomplish this disruptive shift on our own. We will need to closely collaborate with physicians, patients, and other companies with similar ablative technologies.” The future, Kujak believes, will be shaped not only by devices like Vanquish, but by smarter diagnostics, robotics, and interventional imaging platforms that allow for targeted, high-precision therapy.
For Michael Kujak, the work at Francis Medical isn’t just about business or innovation—it’s deeply personal.
“I began my career in pharmaceutical sales, working as a rep in South Dakota before moving into leadership roles at Roche Labs,” he shares. “But it was over two decades ago that I discovered my true passion in medtech—specifically in advancing urologic care.”
The company itself was founded in honor of Francis Hoey, the father of the inventor and a prostate cancer patient who endured harsh treatments before passing away. That legacy still guides the team’s focus on quality of life..
“At Francis Medical, this mission is both professional and personal,” Kujak says. “We’re committed to creating therapies that are tough on cancer and gentle on patients.”











Comments
No Comments Yet!